Site-directed mutation modified algin lyase mutant and application thereof
A technology of alginate lyase and site-directed mutation, which is applied in the field of alginate lyase mutants to achieve the effects of improving catalytic efficiency, improving enzyme production capacity, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Simulation of crystal structure of alginate lyase derived from Escherichia coli
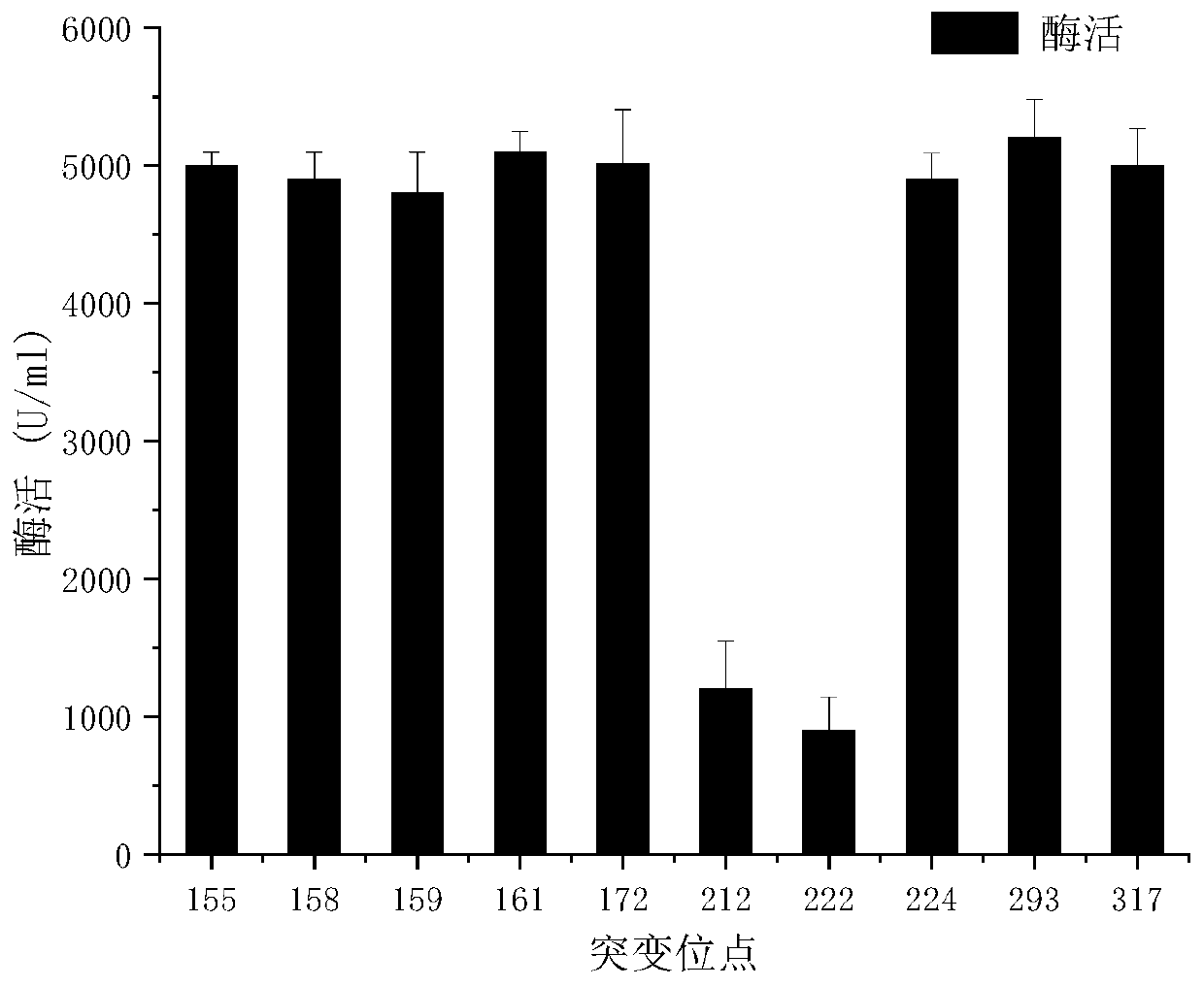
[0035] Using the reported alginate lyase AlyA5 alginate lyase (PDB code: 4BE3) as a template (crystal structure of the exolytic PL7 alginate lyase AlyA5 from Zobelligalactanivorans, published in 2013) (the amino acid similarity between the two is 38.2%), using molecular simulation Docking software that simulates the crystal structure of E. coli-derived alginate lyase. The 212th glutamic acid and the 222nd arginine near the flower-activating domain of the alginate lyase were selected as the active sites of the alginate lyase in this study after the 3D structure simulation docking of the protein in Example 1.
Embodiment 2
[0036] Example 2: The effect of the active site mutation of alginate lyase on the expression of the enzyme activity of alginate lyase
[0037] Using the site-directed mutagenesis kit (TaKaRa), design primers 212A-F, 212A-R, 222A-F, 222A-R (as shown in Table 1), and use the constructed pET-28a (+) as a template to perform PCR. The 212th glutamic acid and the 222nd arginine of alginate lyase were replaced with alanine respectively. The PCR reaction conditions were: 98°C for 5min, 34 cycles (98°C for 30S, 57.6°C for 60S, 72°C for 1.5min) , 72°C for 10 min. PCR amplification system: template 1 μL, upstream and downstream primers 1 μL, Prime Star (Premix) DNA 20 μL, ddH 2 O 17 μL. The gel recovery kit was used to purify and recover the PCR product, and the concentration of the recovered product was checked by electrophoresis. Carry out enzyme digestion under the action of Bam HI and Mlu I fast-cutting enzymes, try to use T4 ligase method to ligate the PCR recovered product, tran...
Embodiment 3
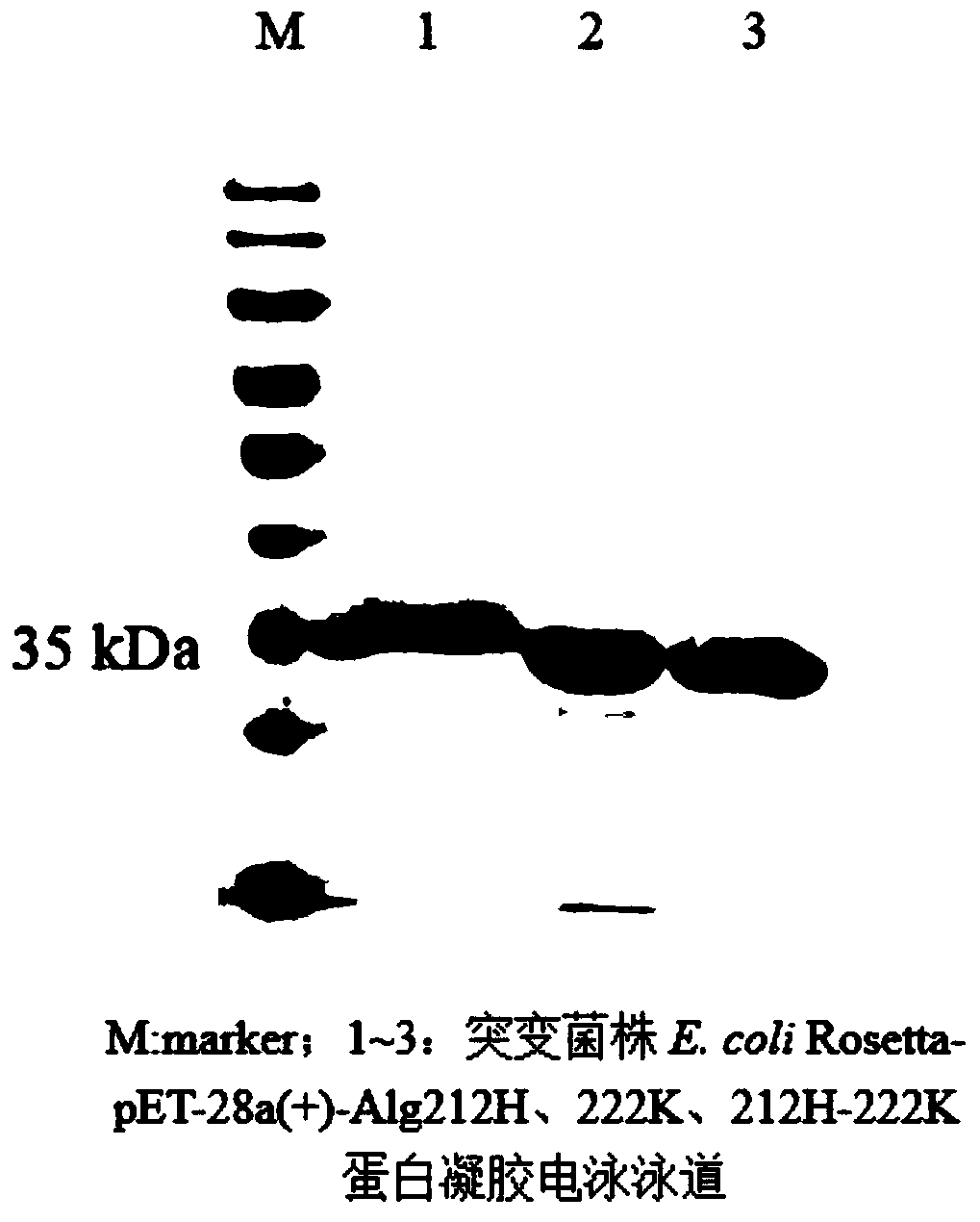
[0040] Example 3: The effect of a single point mutation in the active site of alginate lyase on the expression of the enzyme activity of alginate lyase
[0041] Using the site-directed mutagenesis kit (TaKaRa), design primers 212H-F, 212H-R and 222K-F, 222K-R (as shown in Table 1), and use the constructed pET-28a(+)-Alg212A as a template for PCR , to replace glutamic acid at position 212 or arginine at position 222 by mutation, and the PCR reaction conditions were: 98°C for 5min, 34 cycles (98°C for 30S, 57.8°C for 60S, 72°C for 1.5min), and 72°C for 10min. PCR amplification system: template 1 μL, upstream and downstream primers 1 μL each, Prime Star (Premix) DNA 20 μL, ddH 2 O17 μL. The gel recovery kit was used to purify and recover the PCR product, and the concentration of the recovered product was checked by electrophoresis. Carry out enzyme digestion under the action of Bam HI and Mlu I fast-cutting enzymes, try to use T4 ligase method to ligate the PCR recovered product,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com