Amino acid configuration analysis method and N-polypeptide terminal sequence sequencing method of polymyxin B
A technology for polymyxin and configuration analysis, which can be used in analytical materials, measuring devices, material separation, etc., and can solve problems such as inability to determine amino acid configuration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] This embodiment provides a method for separating and purifying polymyxin B1, polymyxin B2 and polymyxin B1-I from polymyxin B mixed components:
[0087] Take polymyxin B mixed component and add solvent, be mixed with the solution that concentration is 10mg / mL, solvent is water: acetonitrile=80:20 (v / v), adopt liquid chromatography to purify;
[0088] Octadecyl bonded silica gel chromatographic column; injection volume 500 μ L; mobile phase A is water, contains 0.1% formic acid (v / v), mobile phase B is acetonitrile, mobile phase A: mobile phase B=85:15 (v / v); The flow rate is 15mL / min; the ultraviolet absorption wavelength is 215nm; the replenishment liquid is 90% aqueous methanol containing 0.1% formic acid, and the flow rate of the replenishment liquid is 0.45mL / min;
[0089] Select the positive ion scan mode of the electrospray-single quadrupole mass spectrometer, and the mass-to-charge ratio range: 300-1200. Collection of polymyxin B1 (m / z 602.4, [M+2H] 2+ ), poly...
Embodiment 2
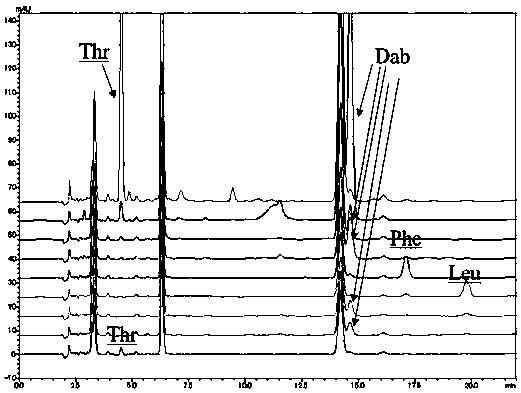
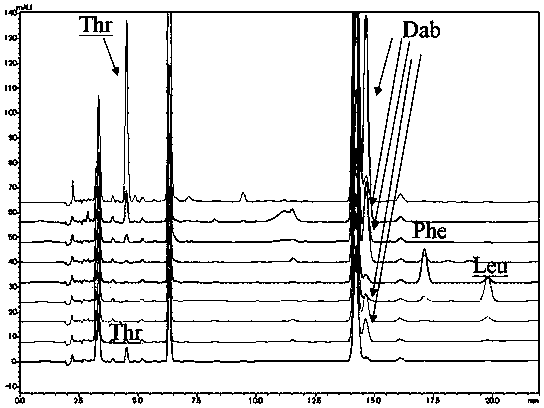
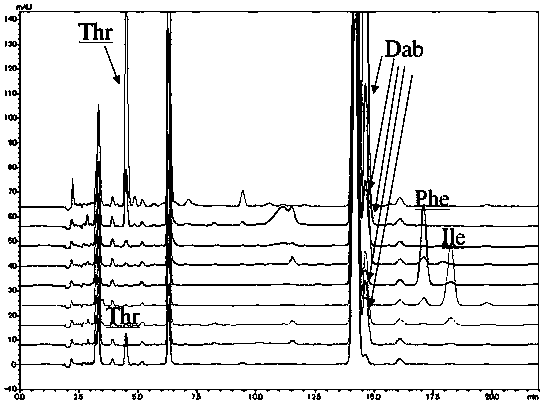
[0094] This embodiment provides a method for analyzing the amino acid configuration of polymyxin B:
[0095]The polymyxin B1, polymyxin B2 and polymyxin B1-I that embodiment 1 is purified are dissolved in the deuterated hydrochloric acid heavy aqueous solution that molar concentration is 6mol / L respectively, make described polymyxin The concentrations of B1, polymyxin B2 and polymyxin B1-I are all 2mg / mL, filled with nitrogen, sealed, pyrolyzed at 110°C for 7h, cooled to room temperature, and added with 6mol / L sodium hydroxide solution respectively , neutralized to neutral, that is, the test solution.
[0096] Measure respectively the reference substance (D / L-leucine, D / L-isoleucine) of polymyxin B1, polymyxin B2 and polymyxin B1-I need testing solution 50 μ L and 0.5 mg / mL amino acid, D / L-phenylalanine, D / L-threonine and D / L-2,4-diaminobutyric acid) solution 30μL, put in the liquid phase sample bottle, add Marfey's reagent solution 50 μL and 0.02-0.08 mol / L triethylamine so...
Embodiment 3
[0111] This example provides the preparation method of polymyxin B1, polymyxin B2 and polymyxin B1-I enzymatic hydrolyzate:
[0112] Take respectively the polymyxin B1, polymyxin B2 and polymyxin B1-150mg that embodiment 1 purifies, be dissolved in the 0.1mol / L potassium dihydrogen phosphate buffer of 7.5mL (pH adjusted by phosphoric acid to 7.0); weigh 12.5mg of ficin and dissolve it in 2.5ml of sodium chloride solution (3mol / L), which is equivalent to 1U / mL of ficin, and incubate in a water bath at 37°C for 30min; mix the above polymyxin B1 , polymyxin B2 and polymyxin B1-I solutions were added to the preheated ficin solution, 37 ° C water bath for 16 hours, boiled for 10 minutes, centrifuged for 10 minutes (eppendorf centrifuge, 13400rpm), filtered to obtain enzymolysis The product solution was used for later use.
[0113] The above enzymatic hydrolysis product solution was used to prepare a chromatographic column using octadecyl bonded silica gel, mobile phase A was 0.1% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com