Snake venom prothrombin activator and snake venom prothrombin activator-based fast hemostatic material
A prothrombin, hemostatic material technology, applied in drug delivery, application, medical science and other directions, can solve the problems of inflammation and tissue necrosis, complex preparation process, skin and other soft tissue damage, etc., to prevent secondary bleeding and infection, experimental The conditions require simplicity, the effect of maintaining activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
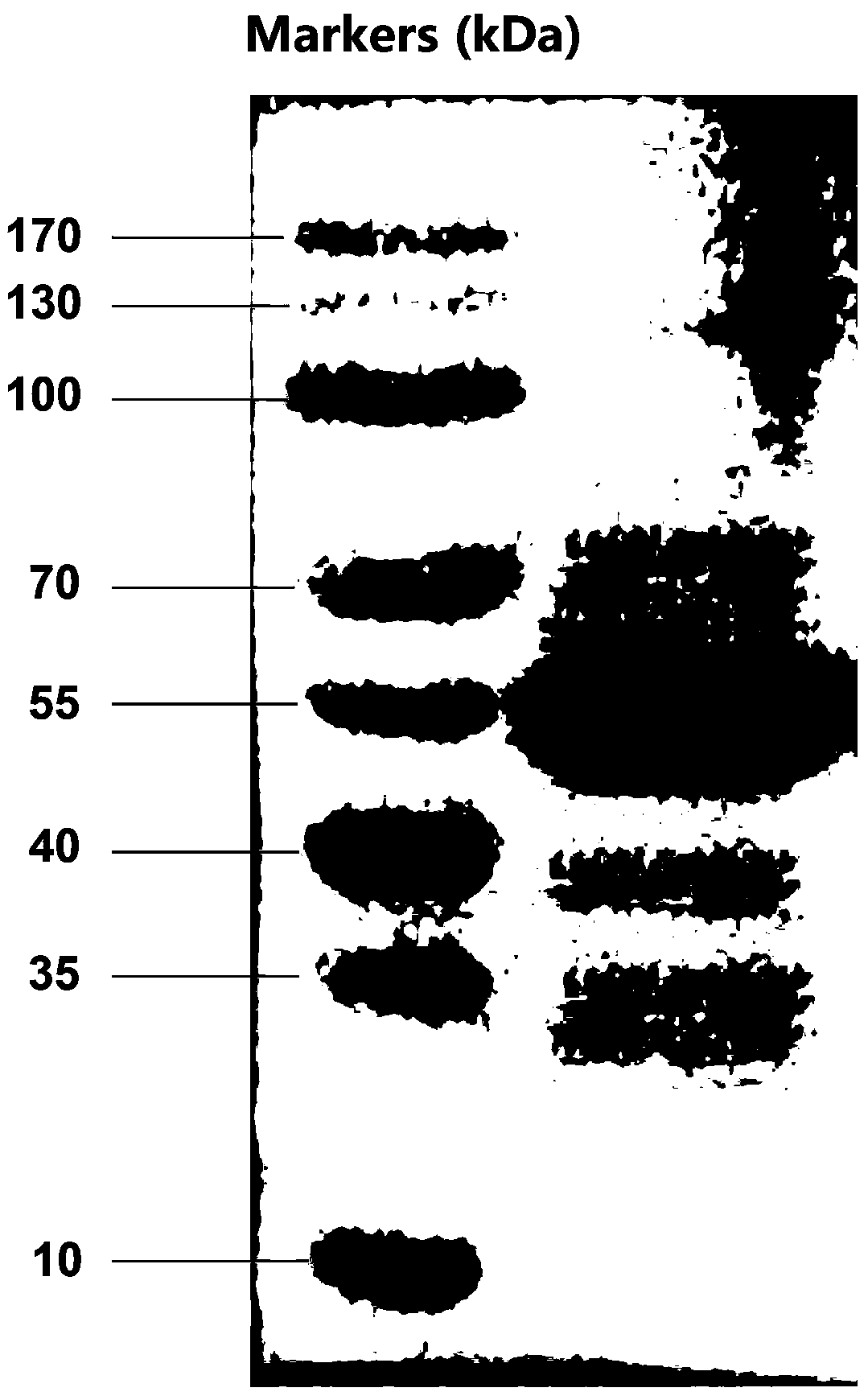
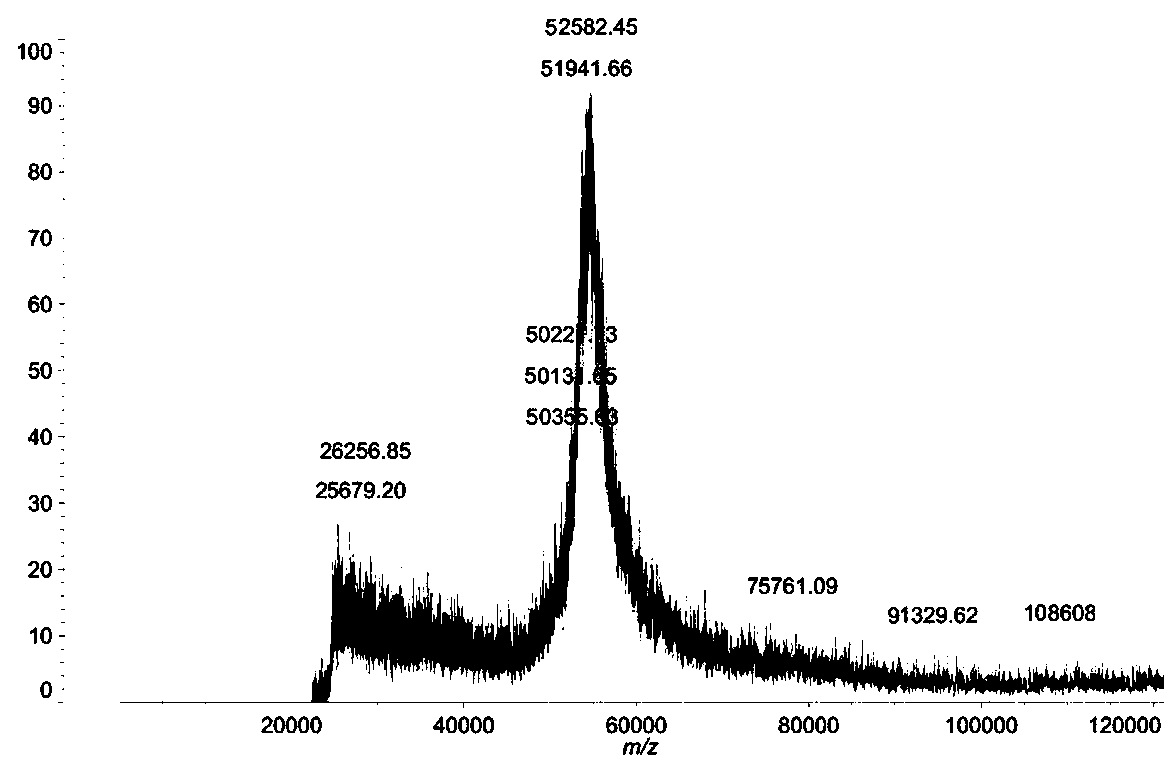
[0054] According to the preparation method of snake venom prothrombin activator of the present invention, 70-100 mg of snake venom prothrombin activator can be obtained per 10 g of snake venom freeze-dried powder.
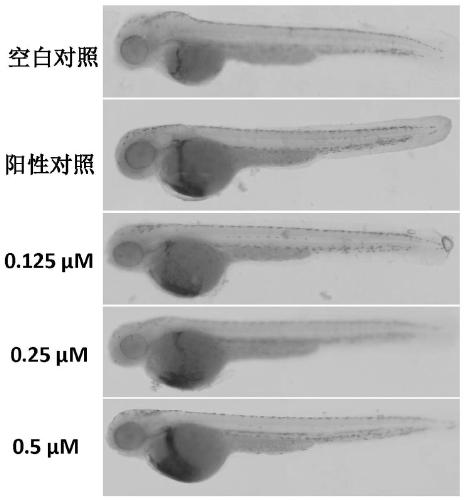
[0055] The present invention also includes a rapid hemostatic material based on snake venom prothrombin activator, which consists of the following raw materials in parts by weight: 0.01-0.5 parts of snake venom prothrombin activator, 5-10 parts of graphene oxide, and 80 parts of polylactic acid ~100 parts, 3~8 parts of modified chitosan, 8~10 parts of calcium chloride, 2~6 parts of nano titanium dioxide and 1~5 parts of polyvinylpyrrolidone;
[0056] Described modified chitosan is prepared according to the following steps:
[0057] Add the dicarboxylic acid into the aqueous sodium chloride solution with a concentration of 0.4-0.6mol / L, start stirring, add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and N- Hydroxysuccinimide, stirred and reacted at 0...
Embodiment 1
[0075] A snake venom prothrombin activator, prepared by the following steps:
[0076] ①Dissolve the lyophilized powder of snake venom in 0.05mol / L PBS buffer solution, filter, and separate and purify the obtained filtrate with a gel column, the filler is Sephadex G-200, first elute with the first mobile phase for 5 hours; The two mobile phases were eluted for 3 hours; the eluent was detected by ultraviolet spectroscopy, and the detection wavelength was 280nm, and the part with ultraviolet absorption was collected, concentrated and desalted with an ultrafiltration membrane with a molecular weight of 5000 to obtain a concentrated solution;
[0077] The mass ratio of snake venom freeze-dried powder and the PBS buffer solution of 0.05mol / L is 1: 10;
[0078] ② The concentrated solution obtained in step ① is subjected to gel column chromatography, the filler is DEAE Sepharose FF, and the first mobile phase is used for elution for 5 hours, and then the second mobile phase is used fo...
Embodiment 2
[0083] A snake venom prothrombin activator, prepared by the following steps:
[0084]①Dissolve the snake venom freeze-dried powder in 0.05mol / L PBS buffer solution, filter, and the obtained filtrate is separated and purified with a gel column, the filler is Sephadex G-200, and first eluted with the first mobile phase for 8 hours; The two mobile phases were eluted for 5 hours; the eluent was detected by ultraviolet spectroscopy, and the detection wavelength was 280nm, and the part with ultraviolet absorption was collected, concentrated and desalted with an ultrafiltration membrane with a molecular weight of 5000 to obtain a concentrated solution;
[0085] The mass ratio of snake venom freeze-dried powder and the PBS buffer solution of 0.05mol / L is 1: 20;
[0086] ②The concentrated solution obtained in step ① was subjected to gel column chromatography, and the filler was DEAE Sepharose FF, and the first mobile phase was used for elution for 8 hours, and then the second mobile ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com