Preparation method of ratiometric fluorescent probe for detecting cefalexin residues and fluorescent probe prepared by preparation method and application thereof
A ratio fluorescent probe, cephalexin technology, applied in fluorescence/phosphorescence, chemical instruments and methods, measuring devices, etc., can solve the problems of large interference, complicated detection operation of cephalexin, expensive instruments and detection environment, etc., to achieve control The effect of food safety, protection of human health, and fast detection speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Detect the preparation of cephalexin fluorescent probe, comprising the following steps:
[0037] (1) Preparation of carbon dots: 2.0 g Tris and 0.125 g lactose were dissolved in 40 mL of purified water. Under magnetic stirring, the mixed solution was heated to reflux at 100° C. for 24 hours, and the color changed from colorless to yellow or even brown. After cooling to room temperature, neutralize the pH value to 7 with hydrochloric acid, and store at 4°C until use to obtain a blue fluorescent carbon quantum dot solution.
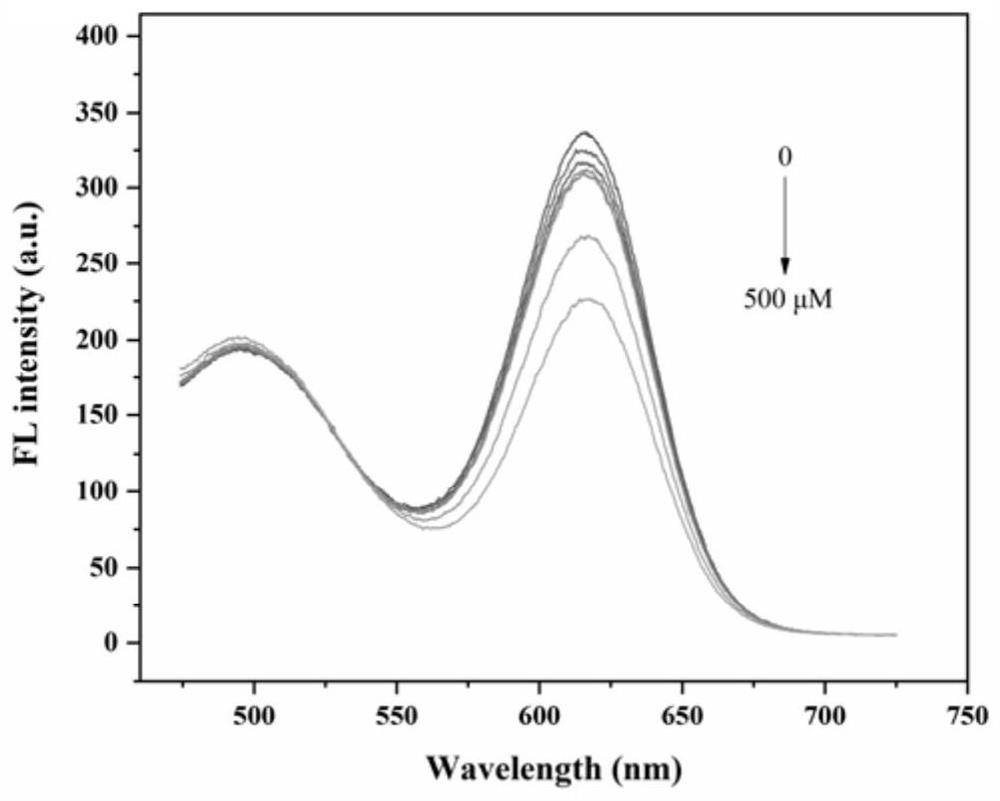
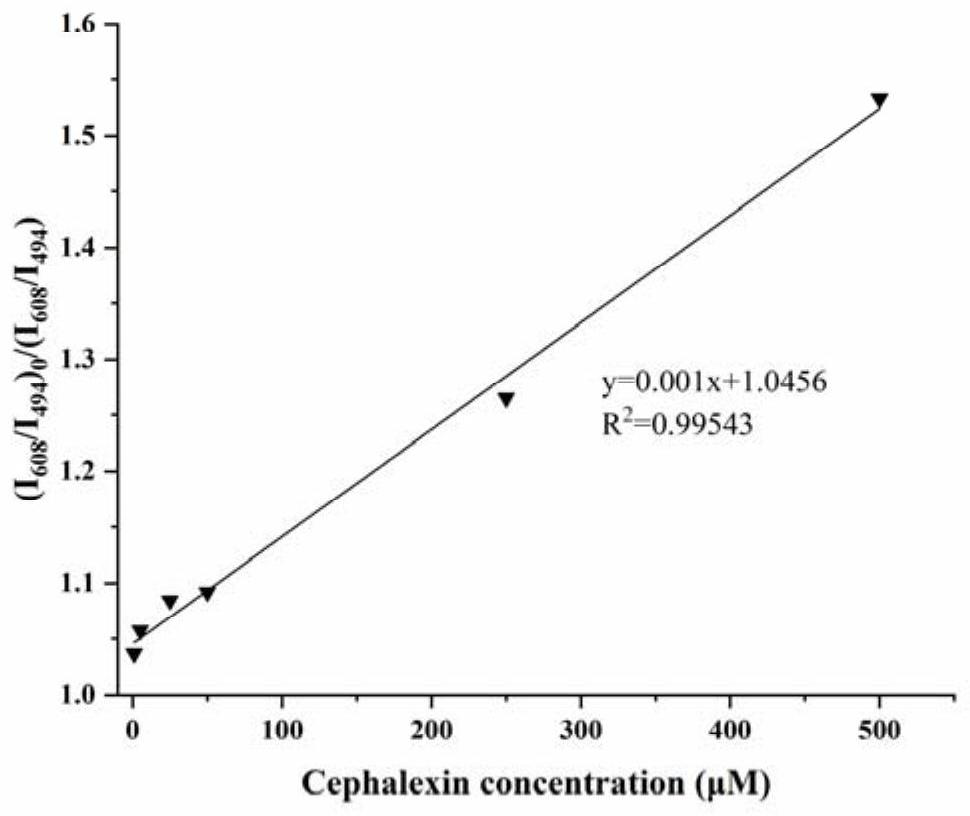
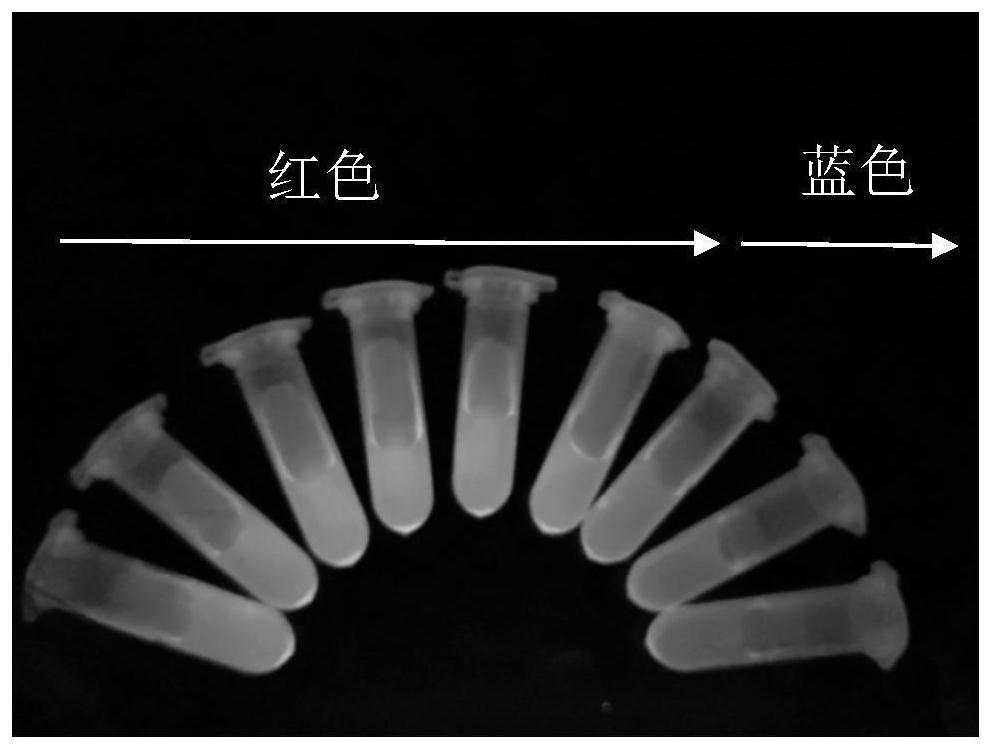
[0038] (2) Preparation of cadmium telluride quantum dots: Add 0.25mmol cadmium chloride, 0.6mmol mercaptopropionic acid, 180mL pure water into a 250mL three-neck round bottom flask, and then adjust the pH to 10 with 1mol / L NaOH. Add 0.0636g Te powder, 0.363g sodium borohydride NaBH to a 10mL round bottom flask 4, and 8mL of pure water, passed through argon and magnetically stirred at room temperature for about 20-30min. When the solution changed fr...
Embodiment 2
[0058] The preparation method of embodiment 2 is identical with embodiment 1, difference is: the mass ratio of Tris described in step (1) and lactose is 15:1; The mol ratio of tellurium powder described in step (2), sodium borohydride is 1:15, reflux reaction at 100°C for 24 hours; the volume ratio of TEOS to APTES in step (3) is 2:1, the volume ratio of carbon quantum dot solution to TEOS is 70:1, and the volume ratio of TEOS to ammonia water is 1:1.5 ; The silicon-coated carbon quantum dot solution and the cadmium telluride quantum dot solution mass ratio of step (4) are 1:3, the cephalexin antibody solution and solution A, the second addition and 1-(3-dimethylamino The volume ratio of the mixed solution of propyl)-3-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide is 3:30:5, and the concentration of the cephalexin antibody solution is 10 ug / mL.
Embodiment 3
[0060] The preparation method of embodiment 3 is identical with embodiment 1, and difference is: the mol ratio of tellurium powder described in step (2), sodium borohydride is 2:3; The carbon quantum dot solution described in step (3) reacts with TEOS The volume ratio is 60:1, and the volume ratio of TEOS to ammonia water is 1:1.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com