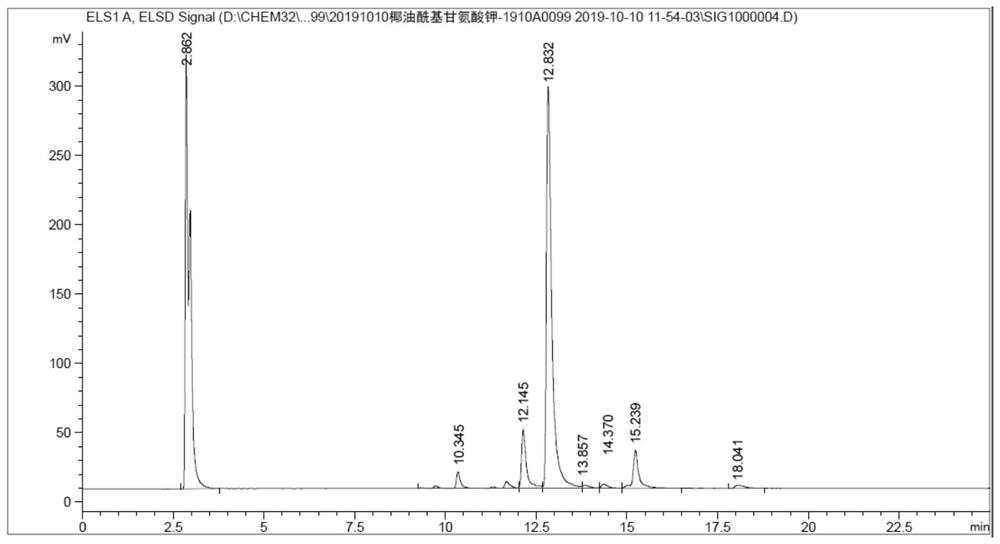
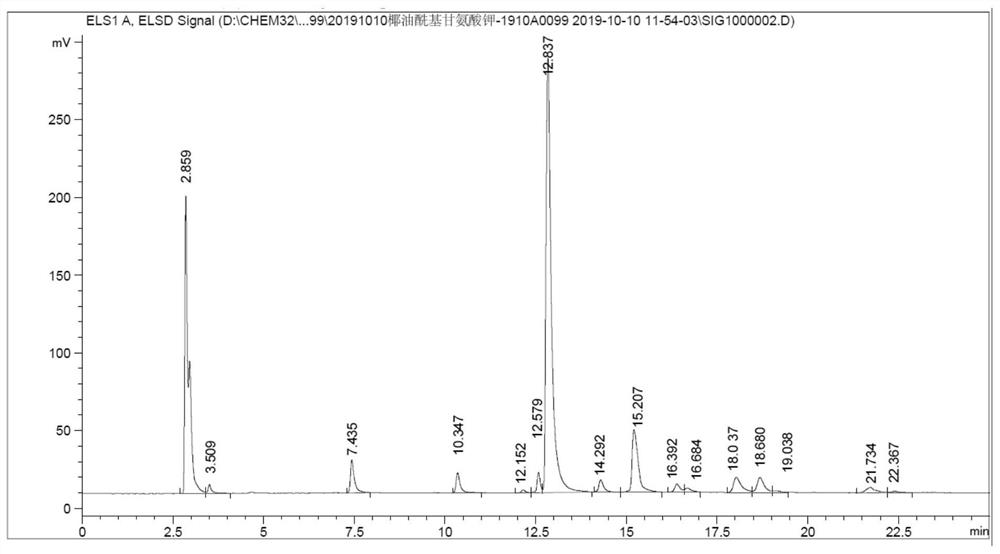
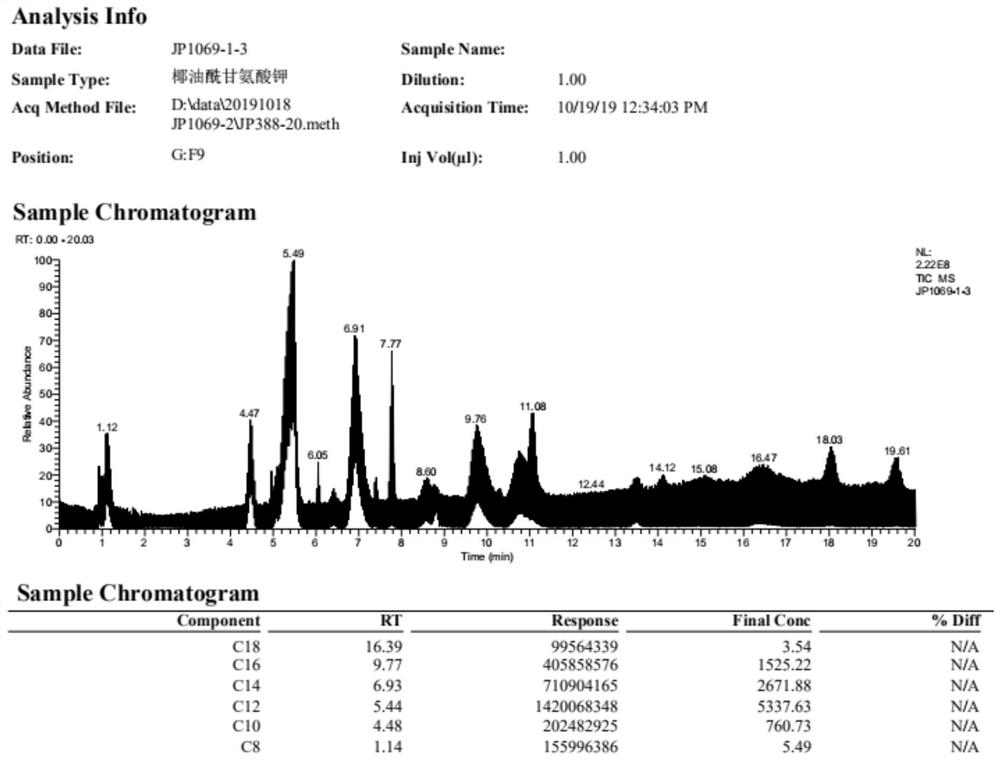
Preparation method of grease acyl amino acid salt
A technology of fatty acid acyl amino acid salt and acyl amino acid salt, which is applied in the field of preparation of fatty acid acyl amino acid salt, can solve the problems of uneven reaction, many by-products, poor color, etc., and achieves avoiding cumbersome post-processing, facilitating industrialization, and simple steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] A preparation method of oleoyl amino acid salt, comprising the following steps:
[0051] Mix glycerol and alkali catalyst, react, prepare mixture A;
[0052]To the mixture A, add monolaurate, oil and amino acid salt, and the temperature is raised to 90°C to 138°C to carry out urethane exchange reaction.
[0053] Adding glycerin to the formula can not only play the role of a dispersant, and improve the uneven stirring during the reaction process and the difficulty of stirring; it can also react with an alkali catalyst to dehydrate the molecules, and the product can promote the subsequent urethane exchange reaction. conduct. In addition to participating in the reaction, monolaurate can also play the role of a penetration enhancer, reducing oil and amino acid salts for urethane exchange While accelerating the reaction process, it cooperates with the alkali catalyst to improve the conversion rate of the reaction; and at a relatively low temperature (90 ° C ~ 138 ° C), the...
Embodiment 1
[0087] The source of the reference substance in Example 1 is Ajinomoto GCK-12H, and the component is potassium cocoyl glycinate.
[0088] Example 1
[0089] This embodiment provides a preparation method of oleoyl amino acid salt and the oleoyl amino acid salt prepared by the method.
[0090] Add 1.8 g of potassium hydroxide and 90 g of glycerol to a 1000 mL four-neck glass bottle, heat to 80 degrees to dissolve, clarify, and remove water with nitrogen for half an hour to obtain mixture A;
[0091] Subsequently, 30.0 g of propylene glycol monolaurate, 150.0 g of coconut oil and 79.24 g of sodium glycinate were added successively to mixture A, heated to 100° C., and reacted for 4 hours. TLC monitored the reaction progress. When the conversion of propylene glycol monolaurate and coconut oil was completed , adding deionized water to dissolve to obtain a clear and transparent sodium cocoyl glycinate solution, then adding hydrochloric acid to adjust the pH value to be about 3, filt...
Embodiment 2
[0107] This embodiment provides a preparation method of oleoyl amino acid salt and the oleoyl amino acid salt prepared by the method.
[0108] Add 1.8 g of potassium hydroxide and 90 g of glycerol to a 1000 mL four-neck glass bottle, heat to 80 degrees to dissolve, clarify, and remove water with nitrogen for half an hour to obtain mixture A;
[0109] Subsequently, 30.0 g of propylene glycol monolaurate, 150.0 g of palm oil and 63.52 g of sodium glycinate were sequentially added to mixture A, heated to 100° C., and reacted for 4 hours. TLC monitored the reaction progress. When the conversion of propylene glycol monolaurate and palm oil was completed , adding deionized water to dissolve to obtain a clear and transparent sodium palmoleoyl glycinate solution, then adding hydrochloric acid to adjust the pH value to about 4, filtering, and vacuum drying at 50 degrees to obtain 210.90g of pale yellow solid palmoleoyl glycine with a yield of 94.79% , and then dissolved with potassium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com