Sustained-release preparation drug-loading material and its composition, sustained-release preparation and preparation method thereof
A technology for sustained-release preparations and compositions, applied in the field of medicine, can solve problems such as difficulty in achieving satisfactory blood drug concentration, small dose, and increase in blood drug concentration, avoiding fluctuations in blood drug concentration, stable drug release, and good biocompatibility. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
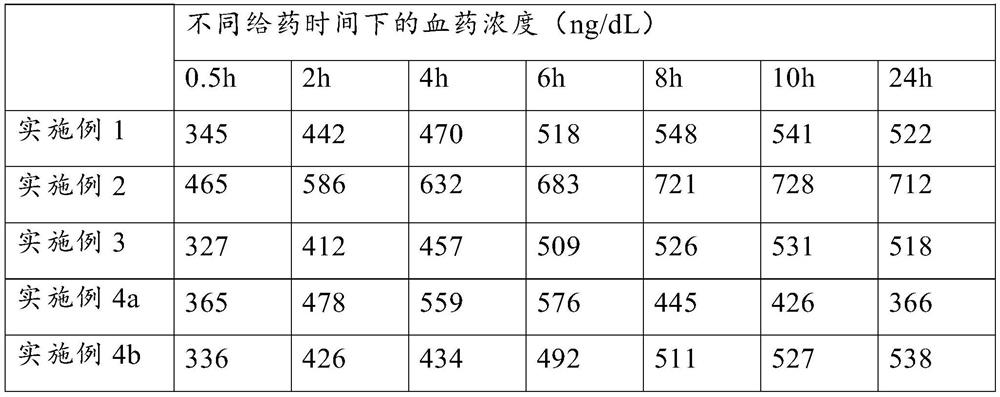
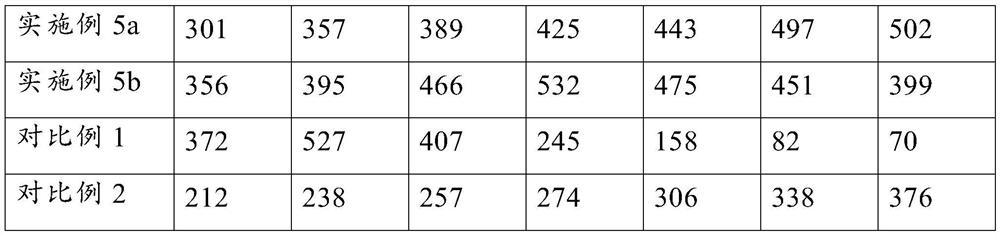
Embodiment 1
[0075] (I) Ingredients
[0076] I1-liposome group:
[0077] Phospholipids: distearoylphosphatidylcholine, 83 parts by weight;
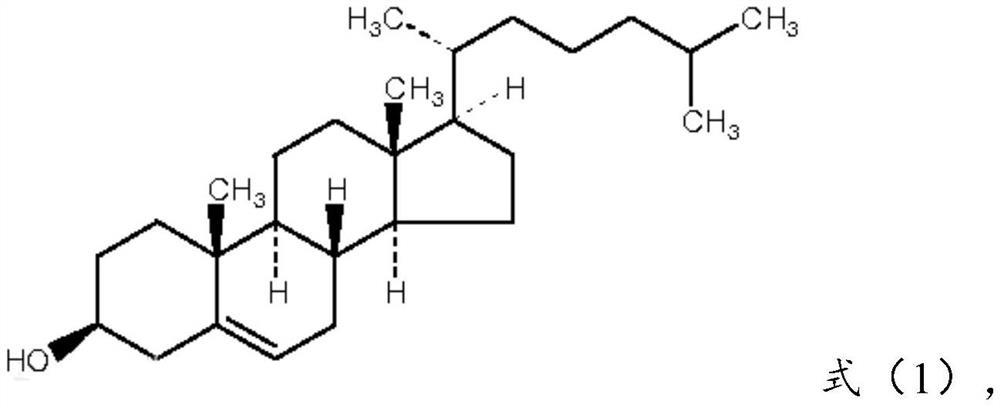
[0078] Cholesterol: compound of formula (1), 17 parts by weight;
[0079] The first penetration enhancer: isopropyl myristate, 187 parts by weight.
[0080] I2-matrix group:
[0081] Aqueous gel compound: hypromellose, 118 parts by weight;
[0082] Fatty acid ester: monoglyceride stearate, 4137 parts by weight;
[0083] The second penetration enhancer: isopropyl myristate, 53 parts by weight.
[0084] I3-Other:
[0085] Medicine: testosterone, 120 parts by weight;
[0086] Solvent: dehydrated alcohol, 500 parts by weight;
[0087] Phosphate buffer: 22200 parts by weight.
[0088] (II) Preparation of anal sustained-release suppositories
[0089] II1-Preparation of matrix: heating and melting the prepared aqueous gel compound, fatty acid ester and second penetration enhancer in a water bath at 100°C to obtain an oil phase;
[0090] II2-Preparat...
Embodiment 2
[0094] (I) Ingredients
[0095] I1-liposome group:
[0096] Phospholipids: egg yolk lecithin, 80 parts by weight;
[0097] Cholesterol: compound of formula (1), 20 parts by weight;
[0098] The first penetration enhancer: N-methylpyrrolidone, 116 parts by weight.
[0099] I2-matrix group:
[0100] Aqueous gel compound: hydroxypropyl cellulose, 81 parts by weight;
[0101] Fatty acid ester: propylene glycol stearate, 2025 parts by weight;
[0102] The second penetration enhancer: N-methylpyrrolidone, 50 parts by weight.
[0103] I3-Other:
[0104] Medicine: dihydrotestosterone, 110 parts by weight;
[0105] Solvent: dehydrated alcohol, 500 parts by weight;
[0106] Phosphate buffer: 22200 parts by weight.
[0107] (II) Preparation of anal sustained-release suppositories
[0108] II1-Preparation of matrix: heating and melting the prepared aqueous gel compound, fatty acid ester and second penetration enhancer in a water bath at 100°C to obtain an oil phase;
[0109] II...
Embodiment 3
[0113] (I) Ingredients
[0114] I1-liposome group:
[0115] Phospholipids: soybean lecithin, 85 parts by weight;
[0116] Cholesterol: compound of formula (1), 15 parts by weight;
[0117] The first penetration enhancer: isopropyl myristate, 169 parts by weight.
[0118] I2-matrix group:
[0119] Water-based gel compound: Carbomer 940, 86 parts by weight;
[0120] Fatty acid ester: polyethylene glycol stearate, 3889 parts by weight;
[0121] The second penetration enhancer: isopropyl myristate, 56 parts by weight.
[0122] I3-Other:
[0123] Medicine: methyltestosterone, 125 parts by weight;
[0124] Solvent: dehydrated alcohol, 500 parts by weight;
[0125] Phosphate buffer: 22200 parts by weight.
[0126] (II) Preparation of anal sustained-release suppositories
[0127] II1-Preparation of matrix: heating and melting the prepared aqueous gel compound, fatty acid ester and second penetration enhancer in a water bath at 100°C to obtain an oil phase;
[0128] II2-Prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com