Synthesis method of furoxan compound
A technology of furoxan and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of dangerous reagents, multiple reactions, and large pollution, and achieve the effects of green and environmental protection, mild reaction conditions, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthesis of 3-cyano-4-aminofuroxan, the structural formula of 3-cyano-4-aminofuroxan is as follows:
[0039]
[0040] The reaction mechanism is:
[0041]
[0042] The synthetic method comprises the steps:
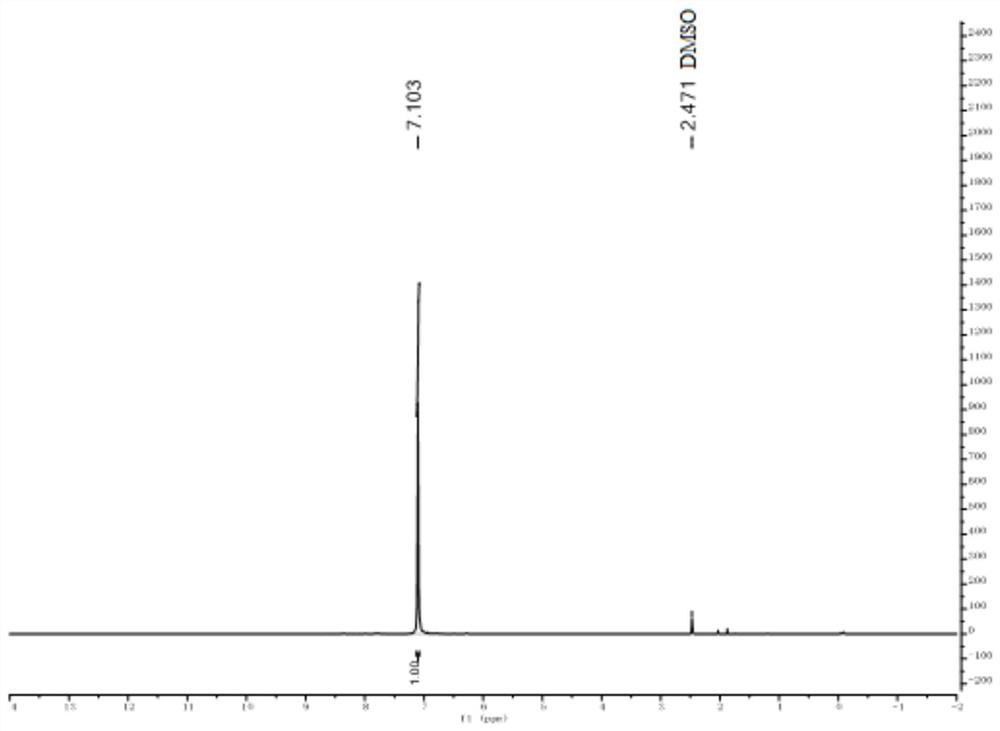
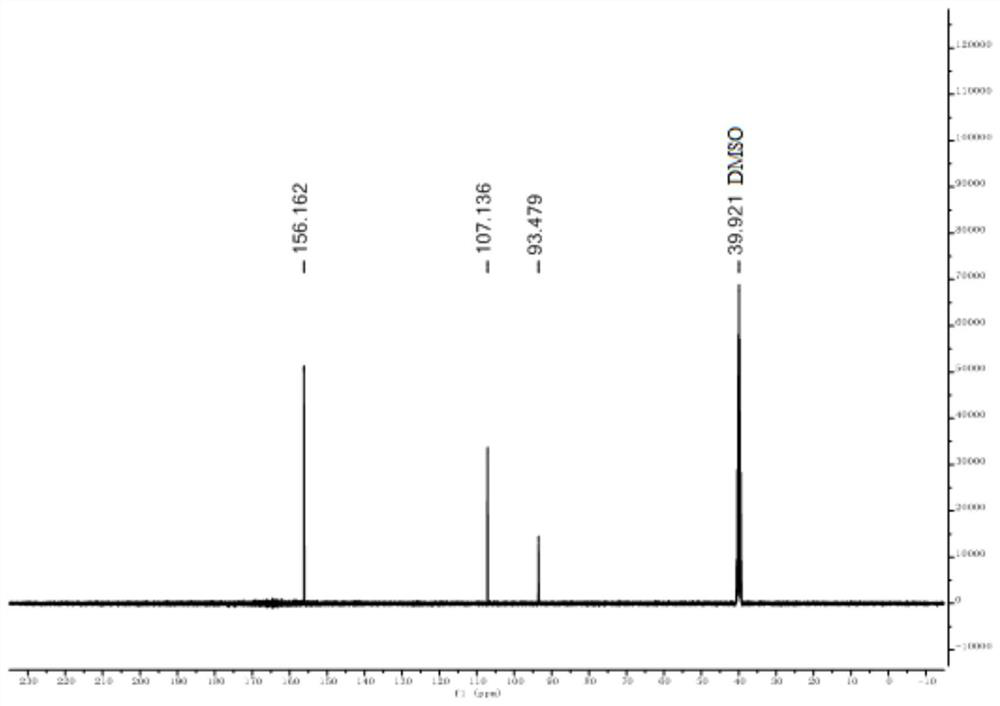
[0043] Take a 250mL three-neck flask, put it into a magnetic stirrer, weigh and add 1-amino-2-cyanoglyoxime (5.12g, 0.04mol), place the reactor in a water bath to maintain the reaction system at 30°C, and then add 120 mL of anhydrous acetonitrile solvent, finally added iodobenzenediethyl ester (12.88 g, 0.04 mol) in batches, and the reaction was stirred at room temperature for 2 h. After the reaction, the organic solvent was removed with a rotary evaporator, washed with ice water, filtered and dried to obtain 3.01 g of white-yellow pure furazan oxide solid particles with a yield of 60%. 1 H NMR (400MHz, DMSO) δ7.10; 13 C NMR (101 MHz, DMSO) δ 156.16, 107.14, 93.48. Anal calcd for C 3 h 2 o 2 N 2 : C28.57, H1.587, N44.44; Found C28.84, H1.75, N44.91...
Embodiment 2
[0046] Synthesis of 3,4-diphenylfuroxan, the structural formula of 3,4-diphenylfuroxan is as follows:
[0047]
[0048] The synthetic route is:
[0049]
[0050] The synthetic method comprises the steps:
[0051] Take a 100mL three-neck flask, put it into a magnetic stirrer, weigh and add benzildioxime (0.24g, 1mmol), place the reactor in a water bath to maintain the reaction system at 30°C, and then add 30mL of absolute ethanol solvent, The stirring system was milky white, and finally iodobenzenediethyl ester (0.322 g, 1 mmol) was added in batches several times, and the solution quickly turned into a yellow-green clear system, and the reaction was stirred at room temperature for 2 h. After the reaction, use a rotary evaporator to remove absolute ethanol, add ice water to wash and extract with ethyl acetate, separate and retain the organic phase, dry and filter with anhydrous sodium sulfate, continue to remove ethyl acetate by vacuum rotary evaporation, and vacuum dry Af...
Embodiment 3
[0054] The synthesis of cyclohexane and furoxan oxide, the structural formula of cyclohexane and furoxan oxide is as follows:
[0055]
[0056] The synthetic route is:
[0057]
[0058] The synthetic method comprises the steps:
[0059] Take a 250mL three-neck flask, put it into a magnetic stirrer, weigh and add 1,2-cyclohexanedionedioxime (0.142g, 1mmol), place the reactor in a water bath to keep the reaction system at 30°C, and then add 30mL of Water and acetonitrile were used as a solvent, and finally iodobenzenediethyl ester (0.322 g, 1 mmol) was added in batches, and the reaction was stirred at room temperature for 2 h. After the reaction was monitored by thin-layer chromatography, the organic solvent was removed with a rotary evaporator, washed with ice water and extracted with ethyl acetate for multiple times to separate the layers. The solvent was removed by rotary evaporation, and finally vacuum-dried to obtain 0.115 g of a yellow pure furoxan oily product wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com