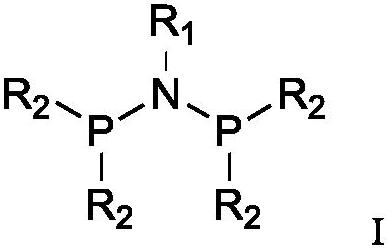
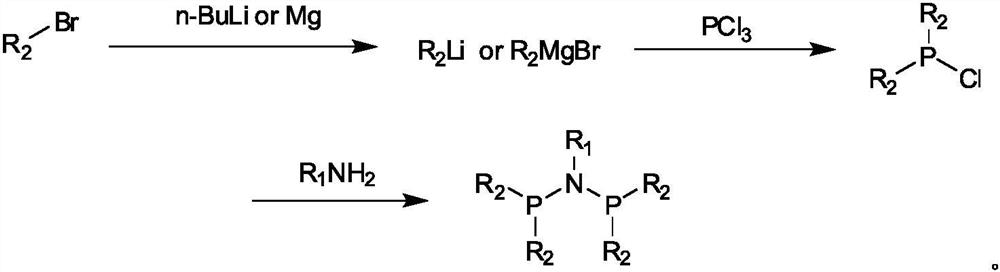
Ethylene oligomerization catalyst system as well as preparation method and application thereof
A technology of ethylene oligomerization and catalyst, applied in the field of ethylene selective oligomerization catalyst system, can solve the problems such as no reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. Synthesis of perfluoronaphthyl PNP ligand
[0055] 1.1, the preparation of 2-bromoheptafluoronaphthalene
[0056]
[0057] Dissolve 25g of octafluoronaphthalene in 150mL of ethanol, stir and heat up to reflux, add 10g of hydrazine hydrate dropwise within 30min, continue to heat and reflux for 4 hours after the dropwise addition, evaporate ethanol at normal pressure, add 200mL of water to the residue, stir and cool to room temperature, The solid was collected by filtration.
[0058] The above solid was added to a mixture of 150mL hydrobromic acid and 20g copper bromide, stirred and heated to reflux for 6 hours, cooled to room temperature, the reaction mixture was extracted three times with 80mL ethyl acetate, the combined organic layers were washed with water, dried, and desolvated, and the obtained solid was used for Methanol was recrystallized to obtain 18 g of yellow solid.
[0059] 1.2. Preparation of PNP ligand
[0060]
[0061] Dissolve 18 g of 2-bromoh...
Embodiment 2
[0073] 1. Synthesis of 5-quinolinyl PNP ligand
[0074] 1.1, Preparation of 5-bromoquinoline
[0075]
[0076] Dissolve 15g of quinoline in 300mL of dichloromethane, add 35g of NBS under stirring at room temperature, and react at room temperature until the reaction of the raw materials is complete, stop the reaction, wash with saturated saline, dry and remove the solvent, and separate by column chromatography to obtain 10.4g of light yellow solid .
[0077] 1.2. Preparation of PNP ligand
[0078]
[0079]Dissolve 10.4 g of 5-bromoquinoline in 50 mL of ether, and add the solution dropwise at room temperature to a suspension of 50 mL of ether containing 1.4 g of magnesium chips, keep stirring, and control the reaction until the magnesium chips disappear completely; 3.4g of phosphorus trichloride was added dropwise to the above mixture at 0°C, then raised to room temperature and stirred for 1 hour, filtered to remove the magnesium salt under the protection of argon, and t...
Embodiment 3
[0090] 1, Synthesis of 6-halazinyl PNP ligand
[0091] 1.1, Preparation of 6-bromothazine
[0092]
[0093] Stir and cool the tetrahydrofuran suspension (350mL) containing 8.2g lithium aluminum hydride to -78°C, slowly add 200mL tetrahydrofuran solution containing 27g 4-bromophthalic acid dropwise within 2 hours, and raise the temperature within 2 hours after the dropwise addition to room temperature, and then heated to reflux for 2 hours. Subsequently, the mixture was cooled to 0°C, and 100 mL of 15% sodium hydroxide solution was slowly added dropwise, vacuum filtered, the filter residue was washed with 200 mL of water and 200 mL of tetrahydrofuran, respectively, the filtrate and washings were combined, and extracted with diethyl ether (100 mL×3), The oil layers were combined, washed with brine, dried and precipitated to obtain 16.4 g of 4-bromo-phthalamide as a white solid.
[0094] The mixed solution of 15mL oxalyl chloride and 180mL dichloromethane was stirred and coo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com