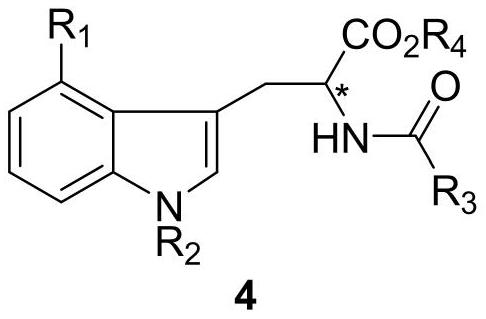
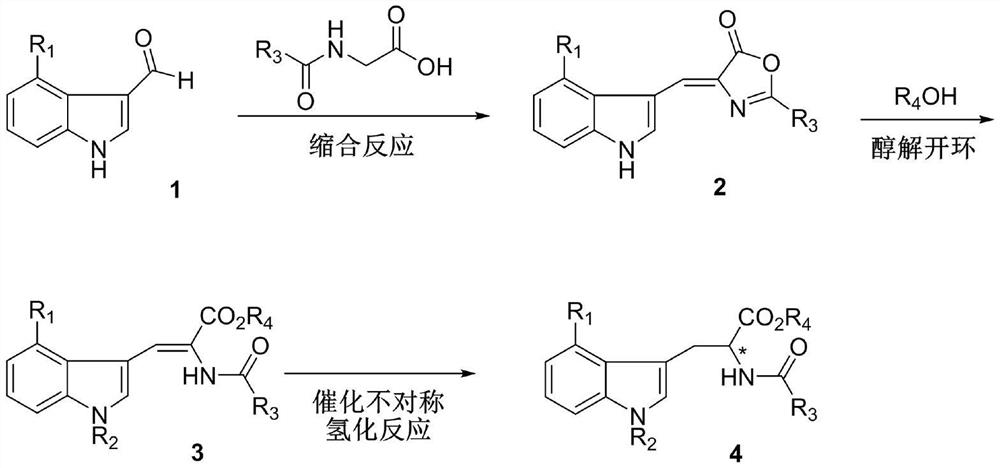
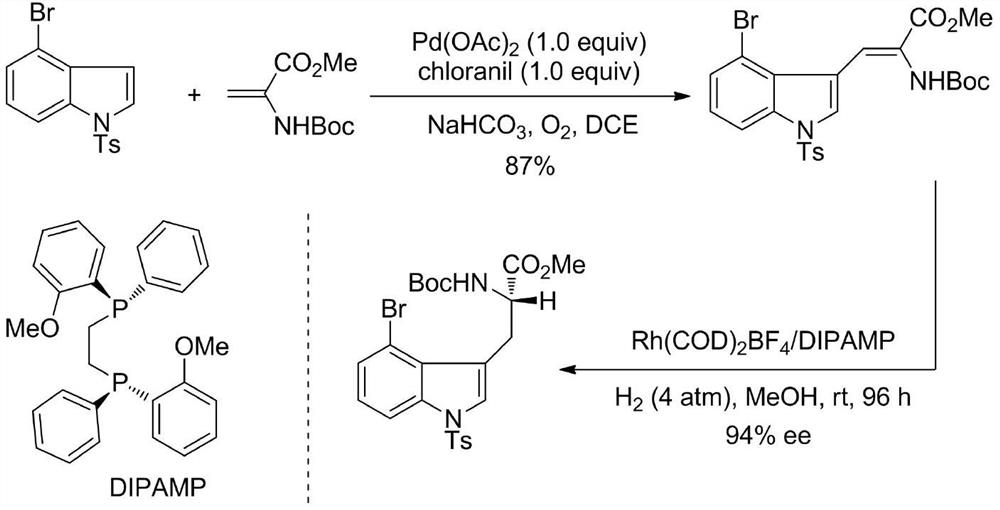
Chiral 4-halogenated tryptophan derivative and synthesis method thereof
A technology for the synthesis of halogenated tryptophan and its application in the field of chiral 4-halogenated tryptophan derivatives and their synthesis, which can solve the problems of low total yield of resolution, long reaction steps, and many impurities in the reaction, etc. , achieve simple and efficient preparation, low cost, and improve reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A synthetic method for chiral 4-bromotryptophan derivatives, comprising the following steps:
[0039]
[0040] S1. In an argon atmosphere, weigh hippuric acid (8g, 0.04mol), sodium acetate (3.7g, 0.04mol) and 27mL acetic anhydride in a 50mL round bottom flask, stir at room temperature for 30min, the reaction mixture turns white Suspension; then weigh 4-bromo-1-hydrogen-indole carboxaldehyde (5g, 0.02mol) and add it to the reaction mixture, stir at room temperature for 1h, then raise the temperature to 65°C and continue stirring for 4h, TLC detects that the raw material has reacted completely (petroleum ether: Ethyl acetate=1:1); add 200mL water to the reaction suspension, and stir at room temperature for 30min, filter the resulting mixture, wash the filter cake with water 3 times (40mL×3), drain, collect the filter cake to obtain 6.7 g compound 2, the yield is 82%.
[0041] Mp 265-267°C; 1 H NMR (400MHz, CDCl 3 )δ9.08(s,1H),8.57–8.50(m,2H),8.12(dt,J=7.1,1.4Hz,2H),7....
Embodiment 2
[0050] A synthetic method for chiral 4-bromotryptophan derivatives, comprising the following steps:
[0051]
[0052] S1. In an argon atmosphere, weigh hippuric acid (8g, 0.04mol), sodium acetate (3.7g, 0.04mol) and 27mL acetic anhydride in a 50mL round bottom flask, stir at room temperature for 30min, the reaction mixture turns white Suspension; then weigh 4-bromo-1-hydrogen-indole carboxaldehyde (5g, 0.02mol) and add it to the reaction mixture, stir at room temperature for 1h, then raise the temperature to 65°C and continue stirring for 4h, TLC detects that the raw material has reacted completely (petroleum ether: Ethyl acetate=1:1); add 200mL water to the reaction suspension, and stir at room temperature for 30min, filter the resulting mixture, wash the filter cake with water 3 times (40mL×3), drain, collect the filter cake to obtain 6.7 g compound 2, the yield is 82%.
[0053] S2. Dissolve compound 2 (6.7g, 0.018mol) in 50mL of methanol, add 5mL of sodium methoxide sol...
Embodiment 3
[0058] A synthetic method for chiral 4-bromotryptophan derivatives, comprising the following steps:
[0059]
[0060] S1. In an argon atmosphere, weigh hippuric acid (8g, 0.04mol), sodium acetate (3.7g, 0.04mol) and 27mL acetic anhydride in a 50mL round bottom flask, stir at room temperature for 30min, the reaction mixture turns white Suspension; then weigh 4-bromo-1-hydrogen-indole carboxaldehyde (5g, 0.02mol) and add it to the reaction mixture, stir at room temperature for 1h, then raise the temperature to 65°C and continue stirring for 4h, TLC detects that the raw material has reacted completely (petroleum ether: Ethyl acetate=1:1); add 200mL water to the reaction suspension, and stir at room temperature for 30min, filter the resulting mixture, wash the filter cake with water 3 times (40mL×3), drain, collect the filter cake to obtain 6.7 g compound 2, the yield is 82%.
[0061] S2. Dissolve compound 2 (6.7g, 0.018mol) in 50mL of methanol, add 5mL of sodium methoxide solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com