Methylene violet derivative fluorescent probe as well as synthesis method and application thereof
A methylene violet and fluorescent probe technology, applied in the field of fluorescent sensing materials, can solve the problems of surrounding tissue damage, inability to directly act on the target tissue, and partial treatment effect, and achieve good anti-tumor ability, fast speed, and water solubility Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A method for preparing a fluorescent probe of a methylene violet derivative is provided, and the reaction formula is as follows:
[0054]
[0055] Include the following steps:
[0056] (1) Dissolve 4-fluoronitrobenzene (4g, 28mmol), potassium carbonate (7.84g, 54mmol) and piperazine (3.6g, 42mmol) in DMSO (30mL), stir at 70°C for 5h, then add appropriate amount of Ice water, a large number of yellow solids precipitated, suction filtered to obtain yellow solid D.
[0057] (2) The above-mentioned compound D (5g, 16mmol), iron powder (1.8g, 32mmol) and ammonium chloride (2.6g, 48mmol) were dissolved in ethanol (100mL), refluxed at 90°C, monitored by TLC until the raw material point disappeared, After the solution is cooled, add saturated sodium bicarbonate solution, stir well and filter. Extracted with ethyl acetate, evaporated to dryness under reduced pressure, and separated by column chromatography to obtain brown oily liquid E.
[0058] (3) The above compound E (1...
Embodiment 2
[0066] A method for preparing a fluorescent probe of a methylene violet derivative is provided, and the reaction formula is as follows:
[0067]
[0068] Include the following steps:
[0069] (1) Methylene violet 3RAX (1 g, 2.6 mmol), chloroacetyl chloride (0.33, 2.9 mmol) and triethylamine (provided with basic conditions) were all dissolved in dichloromethane (25 mL), stirred at room temperature for 8 h, Evaporate to dryness under reduced pressure, and separate by column chromatography to obtain a purple solid A 1 .
[0070] (2) the above compound A 1 (1g, 2mmol), piperazine (0.38g, 4mmol) and potassium carbonate (weak base conditions) (1.2g, 9mmol) were all dissolved in acetonitrile (30mL), stirred at room temperature for 2h, evaporated to dryness under reduced pressure, ethyl acetate Ester extraction, separation by column chromatography, to obtain a red solid B 1 .
[0071] (3) the above compound B 1 (1g, 2mmol), NBD-Cl (0.6g, 3mmol) and triethylamine (provided wit...
Embodiment 3
[0074] A method for preparing a fluorescent probe of a methylene violet derivative is provided, and the reaction formula is as follows:
[0075]
[0076] (1) Add methylene violet 3RAX (1g, 2.6mmol) to concentrated hydrochloric acid and stir for 3h at room temperature, then add 1-phenylpiperazine (0.64g, 4mmol) to the concentrated hydrochloric acid solution of methylene violet , After continuing to stir for 4h, suction filtration gave blue solid C.
[0077] (2) The above-mentioned compound C (1g, 2mmol), NBD-Cl (0.54g, 3mmol) and triethylamine (provided with basic conditions) were all dissolved in dichloromethane (20mL), stirred at 40°C for 15h, and then Evaporate to dryness under pressure and separate by column chromatography to obtain a blue-green solid, which is the fluorescent probe of the methylene violet derivative, which is denoted as 3RAX-N-NBD-3 probe.
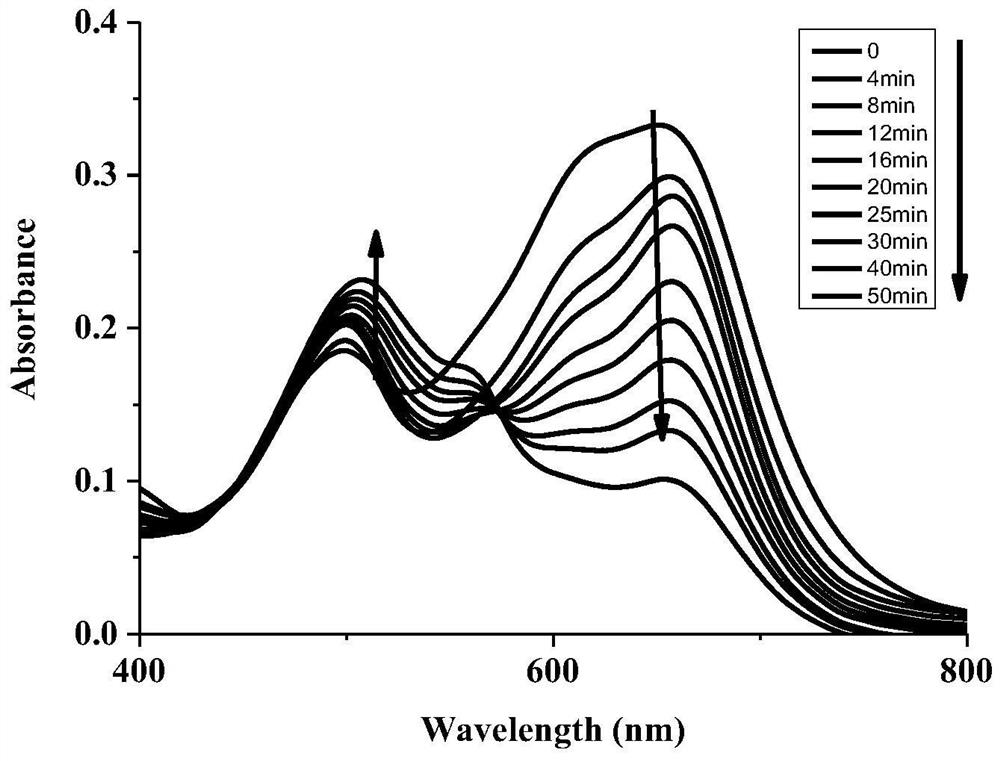
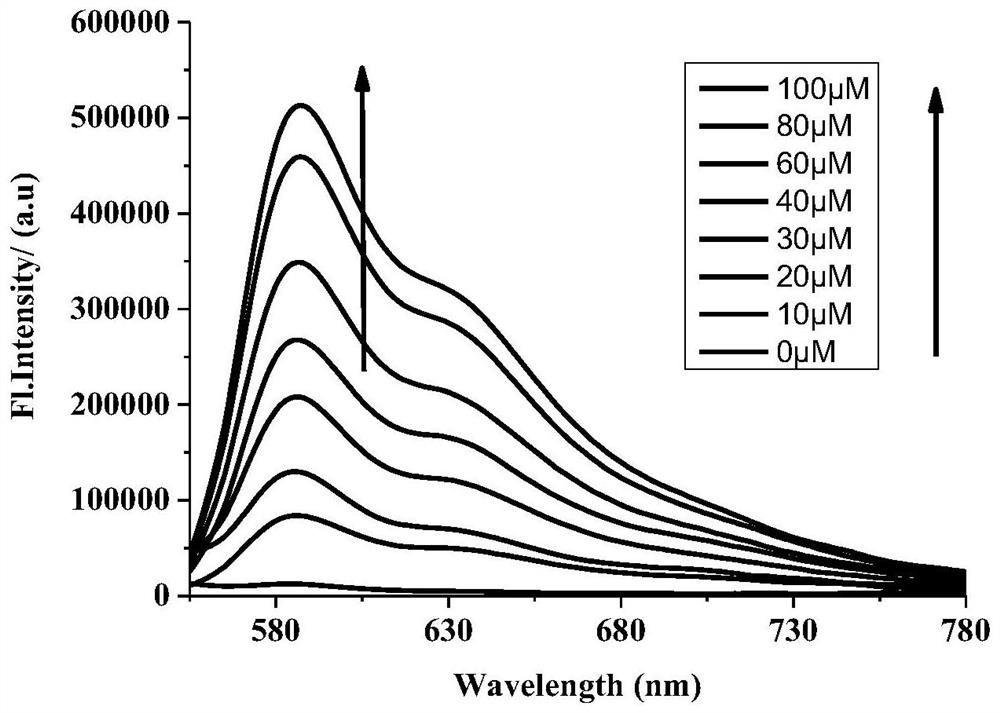
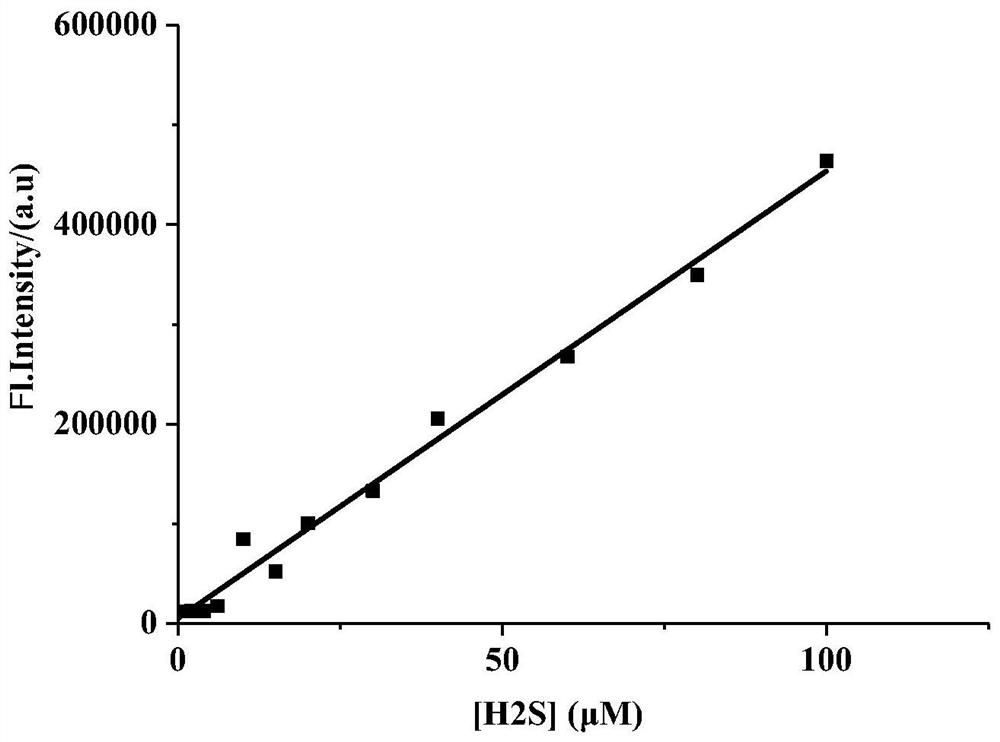
[0078] different concentrations of Na 2 The linear curve (R=0.9908) obtained after the interaction of S with th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com