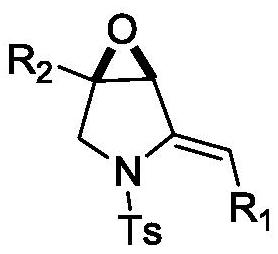
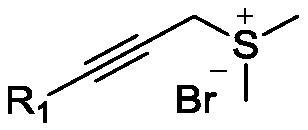
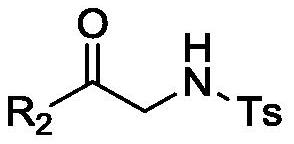
Epoxy-fused 2-methylene pyrrolidine compound and preparation method thereof
A methylene pyrrolidine and compound technology, which is applied to the field of epoxy-fused 2-methylene pyrrolidine compounds and their preparation, can solve the problems of unobtainable raw materials, complicated post-processing process and the like, and achieve convenient post-processing. , simple and easy synthesis method, the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: (1R, 5S / 1S, 5R)-4-methylene-1-phenyl-3-toluenesulfonyl-6-oxa-3-azabicyclo[3.1.0]hexane
[0035]
[0036] 4-Methyl-N-(2-oxo-2-phenylethyl)benzenesulfonamide (0.5mmol, 1.0 equivalent), cesium carbonate (1.0mmol, 2.0 equivalent) and dimethyl (propane-2- Alkyn-1-yl)sulfonium bromide salt (1.0mmol, 2.0eq) was dissolved in 10mL of dichloromethane and stirred overnight at 0–10°C until the reaction of the raw materials was complete. After the reaction was completed, the reaction system was filtered, and the filtrate was concentrated under reduced pressure to obtain a residue. The residue was separated and purified by silica gel column chromatography (petroleum ether:ethyl acetate=10:1) to obtain a yellow oil with a yield of 86%.
[0037] Yellow oil, yield 86%. 1 H NMR(400MHz,DMSO)δ7.72(d,J=8.3Hz,2H),7.42(s,1H),7.40(s,1H),7.39-7.33(m,5H),5.20(s,1H) , 4.92 (s, 1H), 4.35 (d, J = 12.4Hz, 1H), 4.26 (s, 1H), 4.18 (d, J = 12.4Hz, 1H), 2.39 (s, 3H). 13 C NMR (100 MHz,...
Embodiment 2
[0051] Example 2: (1R, 5S / 1S, 5R)-4-methylene-1-phenyl-3-toluenesulfonyl-6-oxa-3-azabicyclo[3.1.0]hexane
[0052]4-Methyl-N-(2-oxo-2-phenylethyl)benzenesulfonamide (0.1mmol, 1.0 equivalent), cesium carbonate (0.15mmol, 1.5 equivalent) and dimethyl (propane-2- Alkyn-1-yl)sulfonium bromide salt (0.15mmol, 1.5eq) was dissolved in 1mL of acetonitrile and stirred overnight at 20°C until the reaction of the raw materials was complete. After the reaction was completed, the yield was calculated by HPLC analysis, and the yield was 45%. In addition to acetonitrile, the solvent can also be any one of toluene, 1,4-dioxane, tetrahydrofuran, N,N-dimethylformamide, dimethyl sulfoxide, and methanol.
Embodiment 3
[0053] Example 3: (1R, 5S / 1S, 5R)-4-methylene-1-phenyl-3-toluenesulfonyl-6-oxa-3-azabicyclo[3.1.0]hexane
[0054] 4-Methyl-N-(2-oxo-2-phenylethyl)benzenesulfonamide (0.1mmol, 1.0 equivalent), 1,8-diazabicycloundec-7-ene (0.15 mmol, 1.5 equivalents) and dimethyl (prop-2-yn-1-yl) sulfonium bromide salt (0.15 mmol, 1.5 equivalents) were dissolved in 1 mL of dichloromethane, stirred overnight at 20 ° C until the raw materials reacted complete. After the reaction was completed, the yield was calculated by HPLC analysis, and the yield was 47%. In addition to 1,8-diazabicycloundec-7-ene (DBU), the base may be selected from potassium carbonate, sodium carbonate, sodium hydroxide, sodium ethoxide, pyridine, N,N-diisopropyl Ethylamine (DIEA), Triethylamine, 4-Dimethylaminopyridine (DMAP).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com