Amphiphilic aza-BODIPY near-infrared dye and preparation method thereof
A technology of azafluoroborodipyrrole and near-infrared dyes, applied in azo dyes, organic dyes, chemical instruments and methods, etc., can solve the problems of short ultraviolet absorption wavelength of azafluoroborodipyrrole dyes, and achieve high photothermal Effects of conversion efficiency, structural stability, and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 (1,7-bis(p-dodecyloxy-phenyl)-3,5-bis(p-3,6,9,11-oxalkoxy-phenyl)azafluoroboron pyrrole)
[0067] Compound A1(C 36 h 54 o 7 )
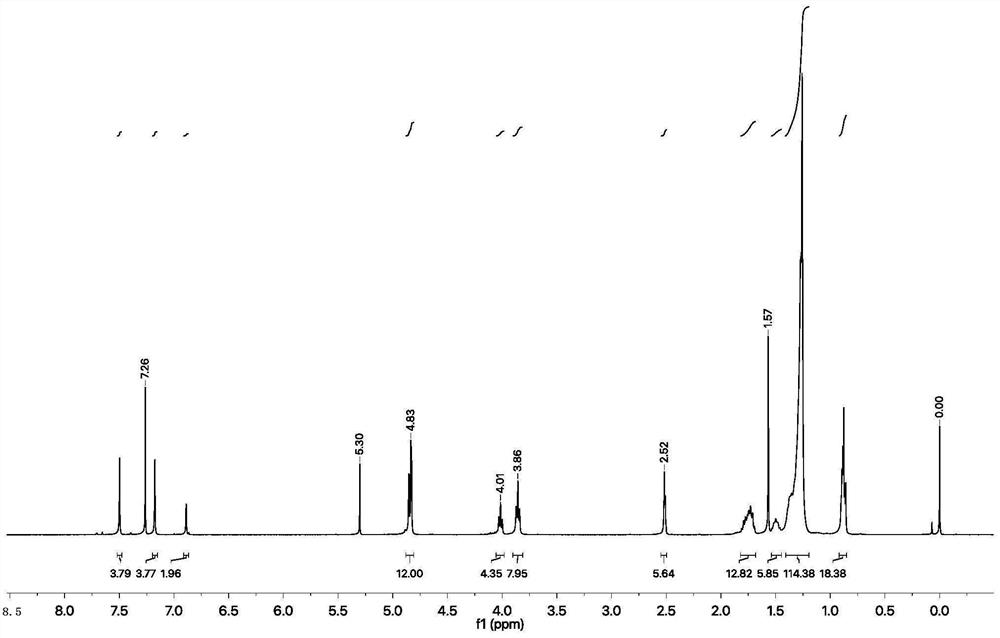
[0068] 4-Dodecyloxybenzaldehyde (0.33mol, 1.1eq) was dissolved in 60mL of ethanol, and 18mL of KOH (0.18mol, 6eq) aqueous solution was added. Then p-3,6,9,11-oxaalkoxyacetophenone (0.03mol, 1eq) dissolved in ethanol (10mL) was added dropwise to the solution, and stirred at room temperature for 24h. The organic solvent was removed by rotary evaporation, dichloromethane was added to dissolve and washed three times with water, the obtained organic solvent was dried with anhydrous sodium sulfate, and the obtained filtrate was filtered to remove the organic solvent by rotary evaporation. The product was purified by silica gel column chromatography and Pet / EtOAc (1:1) to obtain the target product. 1H NMR (400MHz, CDCl3, ppm): δ=8.00(t, J=12.6Hz, 2H), 7.77(d, J=15.6Hz, 1H), 7.59(d, J=8.1Hz, 2H), 7.42( d,J=15.5Hz,1H),6.99(d,J=8.2Hz,2H)...
Embodiment 2
[0079] Example 2 (1,7-two (3,4-two-dodecyloxy-phenyl)-3,5-two (3,4-two-(4'-(N 1 -trioxadecyl)triazole-methoxy)-phenyl)azafluoroborondipyrrole)
[0080] Compound B1
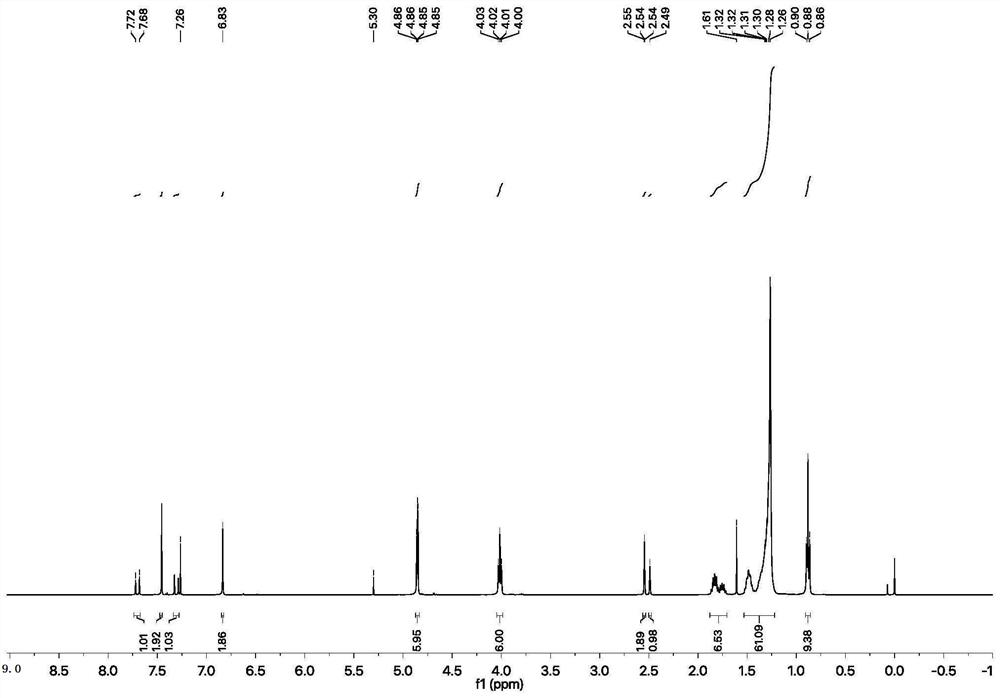
[0081] Add compound 2 (5.5g, 1eq) to the flask, add 20ml of ethanol to dissolve, add KOH aqueous solution (6.17g, 4eq), drop the ethanol solution of compound 3 (13.04g, 1eq) into the flask, and heat up to 50°C Reaction 24h. Detection by thin layer chromatography. After the reaction, filter with suction and dry to obtain the target product. 1H NMR (400MHz, CDCl3) δ7.84–7.66(m,3H),7.35(d,J=15.5Hz,1H),7.21–7.07(m,3H),6.87(d,J=8.3Hz,1H) ,4.84(dd,J=4.0,2.4Hz,4H),4.03(dd,J=6.5,2.4Hz,4H),2.54(d,J=6.3Hz,2H),1.83(dd,J=12.7,7.2 Hz, 4H), 1.47(dd, J=11.5, 3.7Hz, 4H), 1.27(dd, J=16.9, 9.0Hz, 32H), 1.03–0.75(m, 6H). Figure 7 shown.
[0082]
[0083] Compound B2
[0084] Compound 3 (4.6g, 1eq) was dissolved in ethanol, CH3NO2 (2.1g, 5eq) and sodium ethoxide in ethanol (0.06g, 0.1eq) were added to react at 82°C for 12...
Embodiment 3
[0095] Example 3 (1,7-two (3,4,5-three-dodecyloxy-phenyl)-3,5-two (3,4,5-three-(4'-(N 1 -dioxaheptayl)triazole-methoxy)-phenyl)azafluoroborondipyrrole)
[0096] Compound C1(C 60 h 90 o 7 )
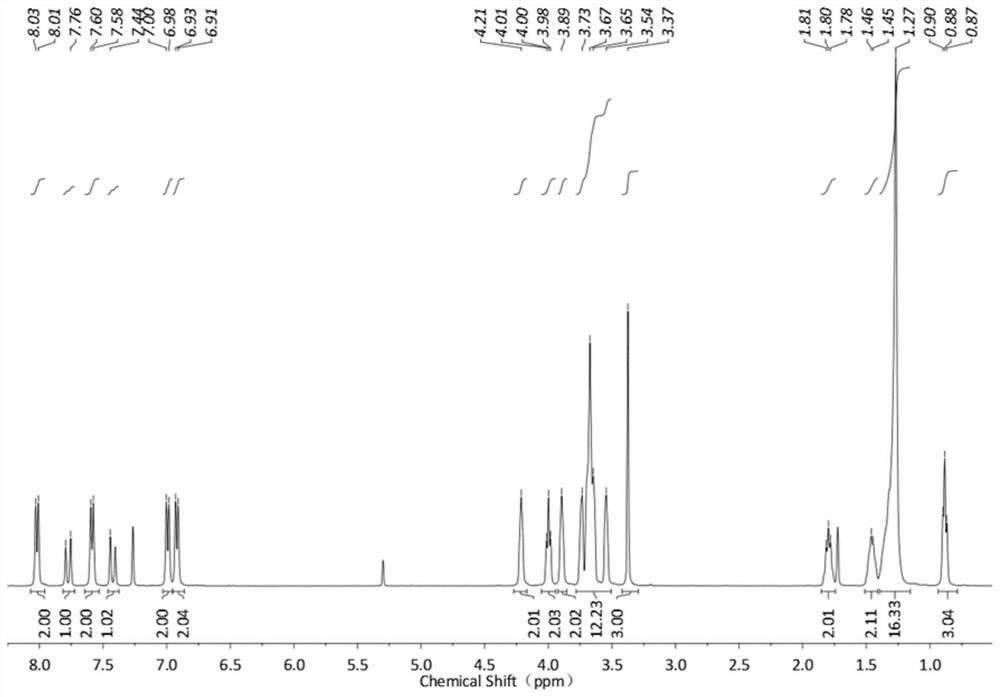
[0097]Synthesis of 3',4',5'-tris(propynyloxy)-3,4,5,-tris(dodecyloxy)-chalcone (C30H38O3): 3,4,5-tripropane Dissolve 15mmol of alkynyloxyacetophenone in a mixed solution of 50ml of ethanol containing 60mmol of potassium hydroxide and 15ml of water, stir for 30min, and slowly drop the ethanol solution containing 15mmol of 3,4,5-tris(dodecyloxy)benzaldehyde Add, react at room temperature for 12h, filter to obtain the precipitate, wash with water to neutral pH, and obtain a white powdery solid. 1H NMR: (400MHz, CDCl3): 8.05(d, J=8.8Hz, 2H), 7.80(d, J=15.6Hz, 1H), 7.60(d, J=8.5Hz, 2H), 7.44(d, J =15.6Hz,1H),7.07(d,J=8.7Hz,2H),6.93(d,J=8.6Hz,2H),4.78(d,J=2.1Hz,2H),3.99(t,2H), 2.56(s,1H)1.80(m,2H),1.46-1.27(m,18H),0.90(t,3H). Figure 13 shown.
[0098]
[0099] Compound C4(C 122 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com