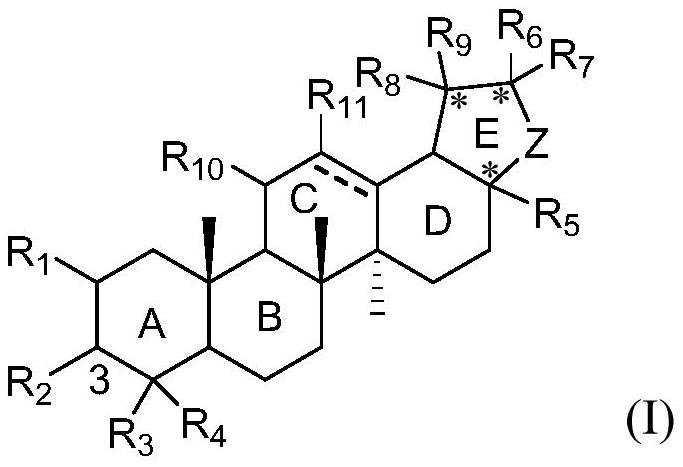
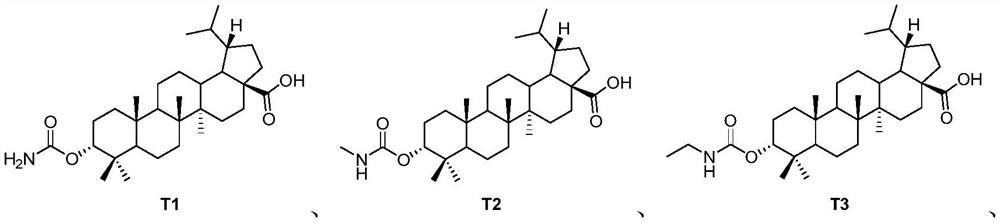
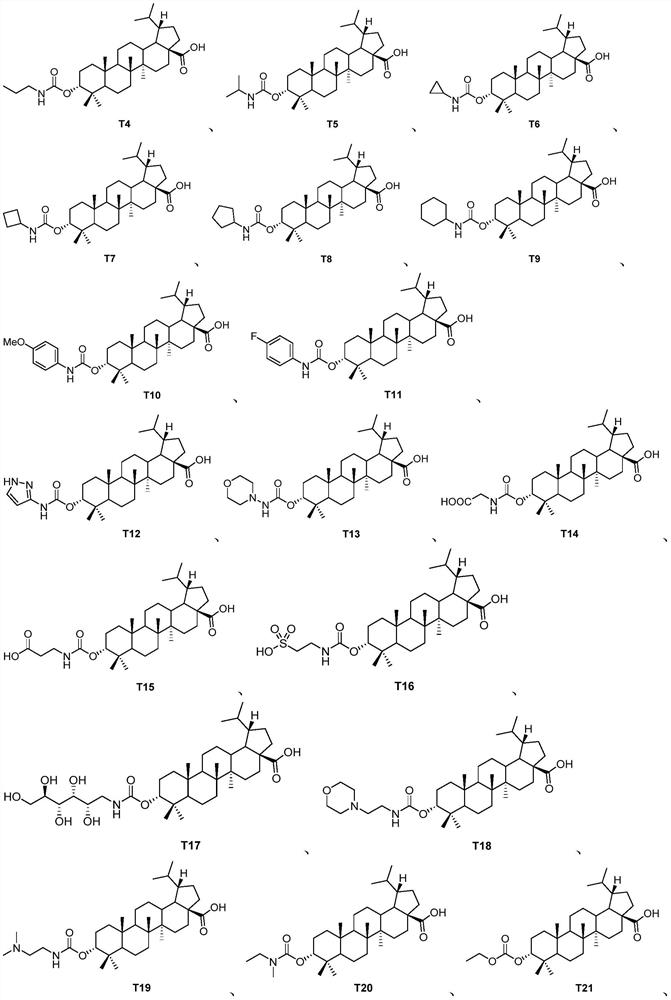
Pentacyclic triterpenoid TGR5 receptor stimulant, preparation method and application thereof
A heterocyclic and cycloalkyl technology, applied in the field of TGR5 agonists, can solve the problems of difficult preparation, insufficient permeability, harsh multi-step reaction conditions, etc., and achieve the effect of abundant natural sources, simple synthesis method, and excellent agonistic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151]
[0152] (1) Dissolve the raw material betulinic acid S1 (1.2g, 2.63mmol) in methanol (50mL) at room temperature. After changing nitrogen, quickly add 10% Pd / C, change nitrogen and then hydrogen, and stir at room temperature. After 24 hours, TLC detected that the reaction was complete. After nitrogen exchange, Pd / C was filtered off, and the reaction solution was spin-dried and separated by column chromatography with petroleum ether / ethyl acetate as an eluent system of 10:1 to obtain 1.04 g (2.27 mmol) of compound S2 as a white solid. Yield: 86%. 1 H NMR (300MHz, CDCl 3 )δ3.13(t, 1H, J=9.0, 6.9Hz), 2.28–2.16(m, 2H), 1.98–1.78(m, 4H), 1.64–0.96(m, other alicyclic protons), 0.96(s ,3H),0.93(s,3H),0.92(s,3H),0.90(s,3H),0.89(s,3H),0.87(s,3H),0.78(s,3H).
[0153] (2) Dissolve the product S2 (1.04g, 2.27mmol) from the previous step in DMF (20mL) at room temperature, add anhydrous potassium carbonate (0.626g, 4.54mmol), and slowly add benzyl chloride (0.313mL ,2.72mmol)....
Embodiment 2
[0159]
[0160] (1) Dissolve S5 (1.00g, 1.82mmol) in dry DCM (10mL), add pyridine (732μL, 9.10mmol) dropwise at 0°C, and add p-nitrophenyl chloroformate (1.10mL) dropwise after 0.5 hours g, 5.46 mmol) in dry DCM (10 mL), returned to room temperature and stirred for 5 hours, TLC showed that the reaction was complete. Spin out DCM, extract with ethyl acetate (3×100mL), wash the combined organic layers with deionized water and saturated brine, dry and concentrate over sodium sulfate, and separate by column chromatography to obtain 1.20g (1.69mmol) of product S7 , Yield: 92.2%. 1 H NMR (300MHz, CDCl 3 )δ8.30-8.27(m,2H),7.40-7.35(m,7H),5.15-5.06(m,2H),4.60(t,1H,J=3.0Hz),2.30-2.15(m,5H) ,2.04-1.05(m, other alicyclic protons),0.96(s,3H),0.95(s,3H),0.92(s,3H),0.86-0.84(m,6H),0.76-0.74(m,6H ).
[0161] (2) Intermediate S-7 (100 mg, 0.182 mmol), DMAP (66.7 mg, 0.546 mmol) and triethylamine (75.7 μL, 0.546 mmol) were dissolved in dry DCM (2 mL), and cyclopropylamine (63.0 μL, 0.9...
Embodiment 3
[0168]
[0169] (1) Preparation of intermediate S8: Sodium hydride (9.84 mg, 0.246 mmol, 60%) was suspended in dry DMF (1 mL), and 2 mL of dry DMF solution of intermediate S8 (76.0 mg, 0.123 mmol) was added dropwise at 0°C , and then kept at 0°C and added dropwise methyl iodide (38.3 μL, 0.615 mmol), returned to room temperature after 10 minutes, and stirred overnight. TLC monitoring the next day showed that the reaction was complete. Quenched by adding deionized water, extracted with ethyl acetate (3×50mL), the combined organic layer was washed with deionized water and saturated brine respectively, dried and concentrated over sodium sulfate, and separated by column chromatography to obtain 59.0 mg of product S9 ( 0.093 mmol), yield: 76.0%. 1 H NMR (300MHz, CDCl 3 )δ7.37-7.30(m,5H),5.15-5.06(m,2H),4.49(t,1H,J=2.7Hz),3.35-3.28(m,2H),2.90(s,3H),2.30 -2.15(m,3H),1.91-0.96(m,other alicyclic protons),0.93(s,3H),0.88-0.84(m,12H),0.76-0.73(m,6H).
[0170] (2) Compound T20 (32....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com