Method for producing N,N'-dicyclohexyl carbodiimide
A technology of dicyclohexylcarbodiimide and dicyclohexylurea, applied in the field of production of N,N'-dicyclohexylcarbodiimide, which can solve the problems of high odor, unstable yield, and insufficient yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Stir 200g of dicyclohexyl urea (DCU) and 800g of ethylene glycol monobutyl ether at room temperature to cool down to about 10°C, add 360g of trichloromethyl carbonate, 2.5g of aluminum trichloride loaded on molecular sieves, and heat up Keep warm at 35°C and reflux for 2 hours, cool down to 5°C, add N,N-dimethylethanolamine dropwise, adjust the pH to 8-9, filter the reaction solution, distill off the solvent, and distill under reduced pressure at 0.9-1.2KPa to obtain the product N, N'-dicyclohexylcarbodiimide, the yield is 92.32%, and the purity is 99.55%.
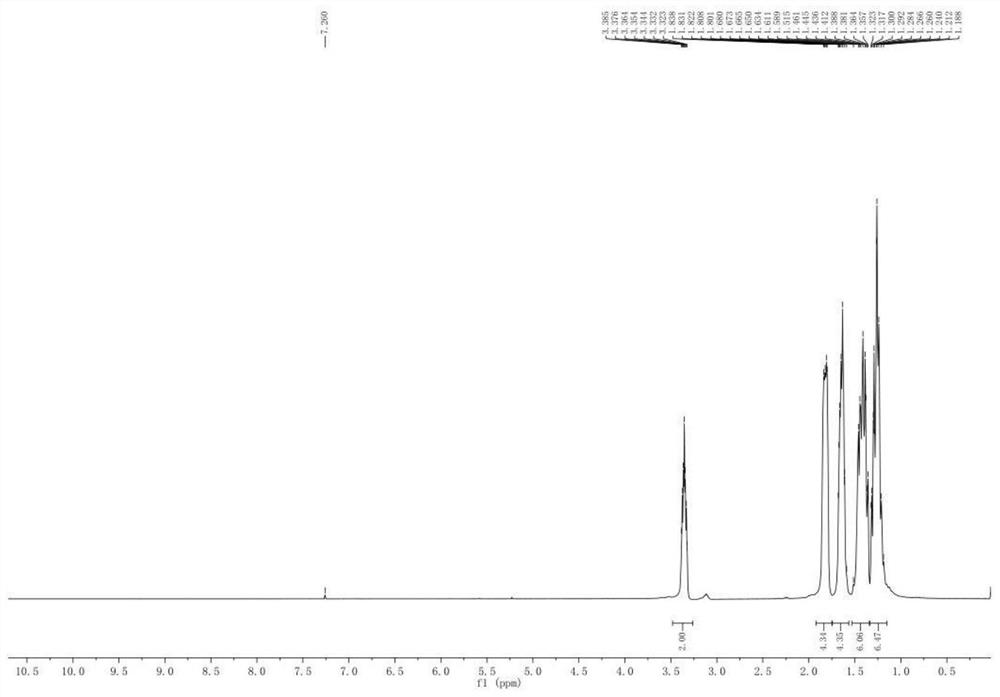
[0028] The product was determined by proton nuclear magnetic spectrum, and the obtained spectrum was as follows: figure 1 shown. Among them: the two hydrogen chemical shifts of 1,1' are 3.323-3.385; the eight hydrogen chemical shifts of 2,2' are 1.515-1.838; the eight hydrogen chemical shifts of 3,3' are 1.284-1.461; 4,4 The four hydrogen chemical shifts of ' are at 1.188-1.292; that is, the structural formula of ...
Embodiment 2
[0031] Stir 200g of dicyclohexyl urea (DCU) and 600g of tolylcyclohexanone at room temperature to cool down to about 10°C, add 220g of trichloromethyl carbonate, 1.5g of ferric chloride loaded on molecular sieves, and heat up to 40°C Insulate and reflux at ℃ for 2 hours, lower the temperature to 5℃, add tri-n-butylamine dropwise, adjust the pH to 8-9, filter the reaction solution, distill off the solvent, and distill under reduced pressure at 0.9-1.2KPa to obtain the product N,N'-dicyclohexyl Carbodiimide, the yield is 89.35%, and the purity is 99.27%.
Embodiment 3
[0033] Stir 200g of dicyclohexyl urea (DCU) and 1000g of ethyl acetoacetate at room temperature to cool down to about 10°C, add 420g of trichloromethyl carbonate, 2.1g of tin tetrachloride loaded on molecular sieves, and heat up to 50 Insulate and reflux at ℃ for 4 hours, lower the temperature to 5℃, add diethylenetriamine dropwise, adjust the pH to 8-9, filter the reaction solution, distill off the solvent, and distill under reduced pressure at 0.9-1.2KPa to obtain the product N,N'-dicyclohexyl Carbodiimide, the yield is 92.54%, and the purity is 99.28%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com