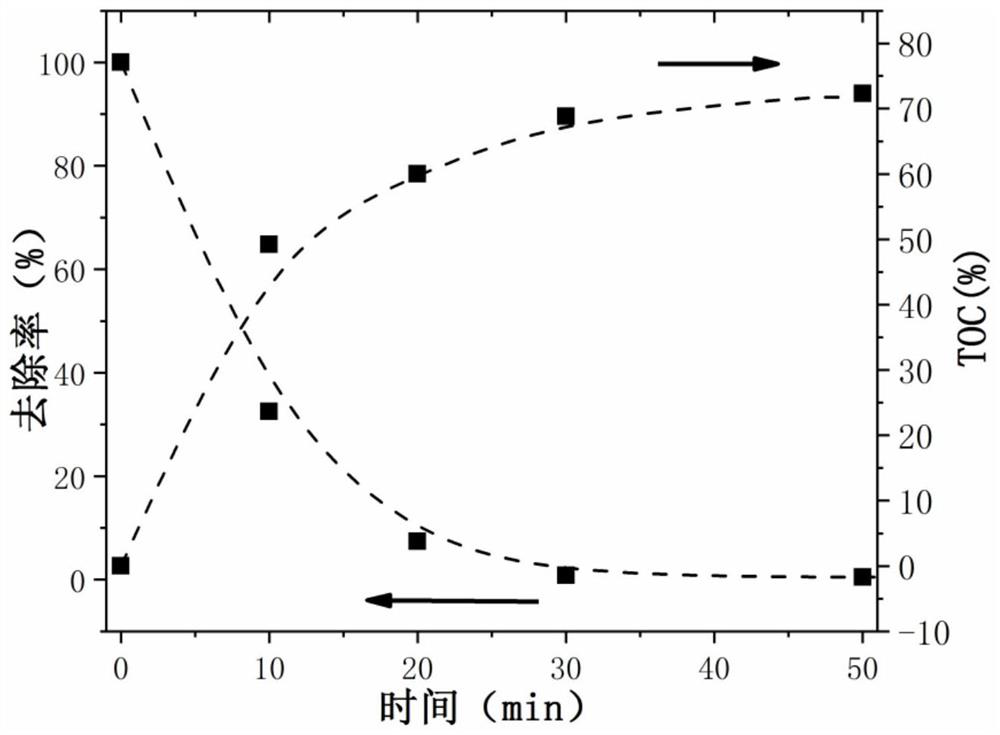
Bimetallic core-shell catalyst for electro-catalysis synergistic hydrogen production as well as preparation method and application of bimetallic core-shell catalyst
A core-shell catalyst, electrocatalysis technology, applied in physical/chemical process catalysts, chemical instruments and methods, electrodes, etc., can solve the problems of long reaction process time, increased processing time, weak superoxide radical activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A method for preparing a core-shell catalyst for electrocatalytic synergistic hydrogen production, comprising the steps of:
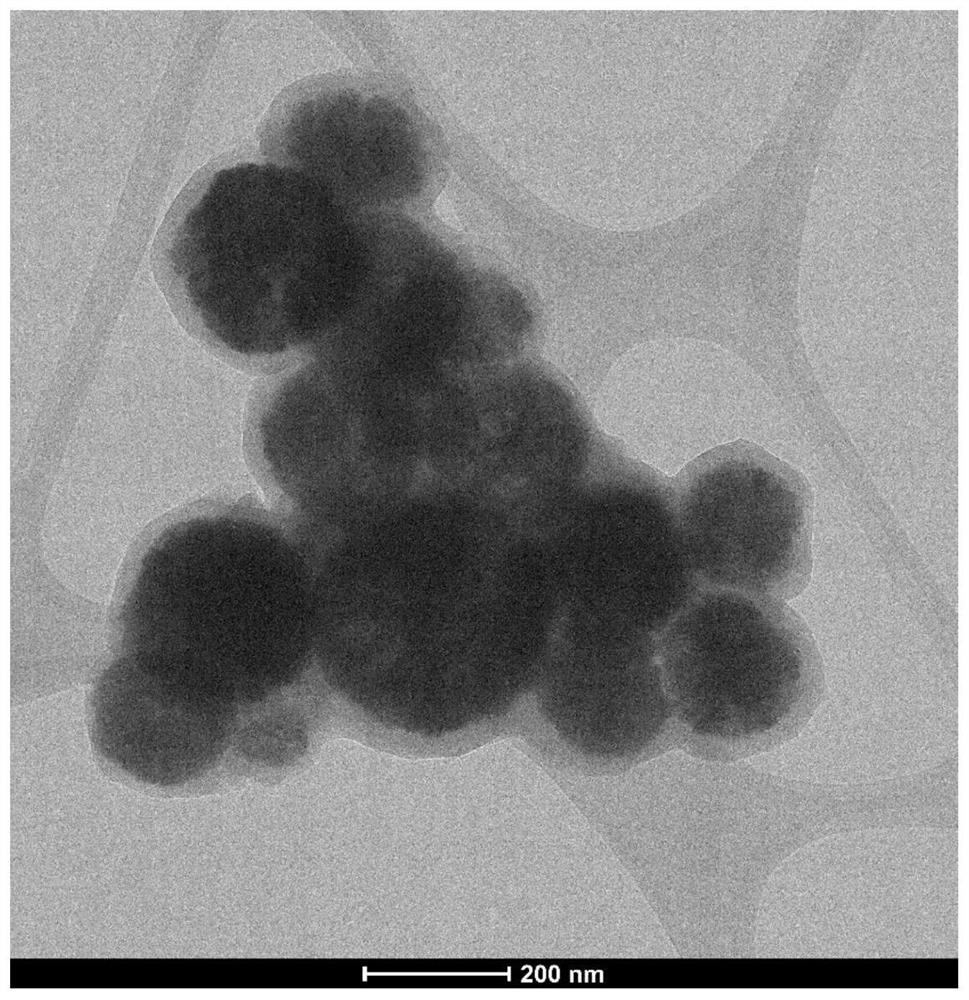
[0044] 1) Add 1.35g FeCl to a 50mL beaker 3 ·6H 2 O, 0.124MnCl 2 4H 2 O and 40mL ethylene glycol, stir vigorously to turn bright yellow, add 3.6gNaAC, stir well, add 10mL ethylenediamine to obtain a dark green solution, pour it into a 50mL polytetrafluoroethylene reactor, and react at a temperature of 200°C for 12h , washed with ethanol three times after cooling and dried in vacuum to obtain the bimetallic MnFe 3 o 4 nanospheres.
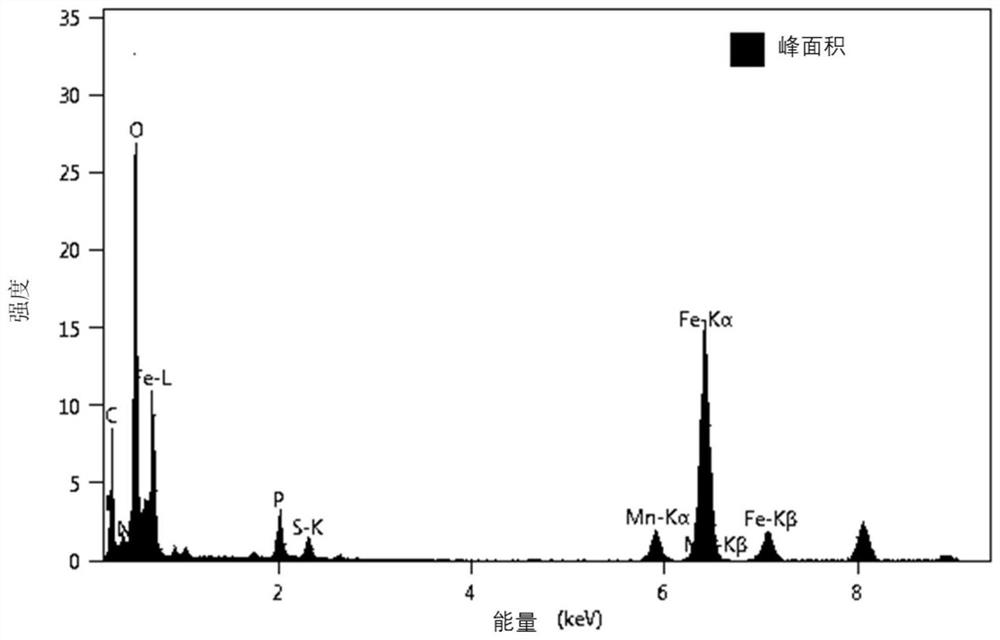
[0045] 2) Using 10 mg of hexachlorocyclotriphosphazene as the source of N and P, 22.5 mg of thioacetamide as the source of S, adding 50 mg of the bimetallic MnFe 3 o 4 Add 15 mL of ethanol and tetrahydrofuran at a mass ratio of 1:1 to the nanospheres as a solvent, and ultrasonicate for 5 min at an ultrasonic frequency of 50 Hz until the reactants are completely dissolved and uniformly dispersed. Then seal it wi...
Embodiment 2
[0056] A method for preparing a core-shell catalyst for electrocatalytic synergistic hydrogen production, comprising the steps of:
[0057] 1) Add 0.628g FeCl to a 50mL beaker 3 ·6H 2 O, 1.0g MnCl 2 4H 2 O and 40mL ethylene glycol, stir vigorously to turn bright yellow, add 3.6gNaAC, stir well, add 10mL ethylene glycol to obtain a dark green solution, pour it into a 50mL polytetrafluoroethylene reactor at 200°C for 12h, wash with ethanol for 3 times and vacuum Dry to obtain bimetallic MnFe 3 o 4 nanospheres.
[0058] 2) The heteroatom shell uses 20mg of hexachlorocyclotriphosphazene as the N source and P source, 45mg of thiourea as the S source, and 50mg of the above-mentioned bimetallic MnFe 3 o 4 Nano-microspheres, 15mL of ethanol and tetrahydrofuran with a mass ratio of 1:1 as a solvent, ultrasonic (50Hz) 5min completely dissolved and uniformly dispersed. Then seal it with a polyethylene film, and quickly inject 3 mL of triethylamine as an acid-binding agent with a ...
Embodiment 3
[0062] A method for preparing a multi-component heterogeneous nodule shell catalyst suitable for electric Fenton, comprising the steps of:
[0063] 1) Add 0.675g FeCl to a 50mL beaker 3 ·6H 2 O, 0.248g MnCl 2 4H 2 O and 40mL polyvinyl alcohol, stir vigorously to turn bright yellow, add 3.6gKAC, stir well, add 10mL ethylenediamine to obtain a dark green solution, pour it into a 50mL polytetrafluoroethylene reactor, react at 200°C for 24h, and ethanol after cooling After washing 3 times and drying in vacuum, the metal microsphere core (MnFe 3 o 4 ).
[0064] 2) Adopt 50mg hexachlorocyclotriphosphazene as N and P source, 112.5mg sulfonyldiphenol as S source, 250mg above-mentioned solvothermal method preparation diameter is the metal microsphere core of 50x50 ± 10nm, 30mL mass ratio is 1: 1 ethanol and tetrahydrofuran as solvents, ultrasonic (100Hz) 5min completely dissolved and uniformly dispersed. Then seal it with plastic wrap, and quickly inject 2 mL of trimethylamine a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com