Crystal face regulation and control preparation method and application of porous manganese dioxide
A manganese dioxide and crystal face technology, which is applied in the field of catalyst preparation, can solve the problems of poor activity and poor stability of selective oxidation catalysts, and achieves the improvement of H2S oxidation reaction activity, stability, adsorption capacity and redox capacity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
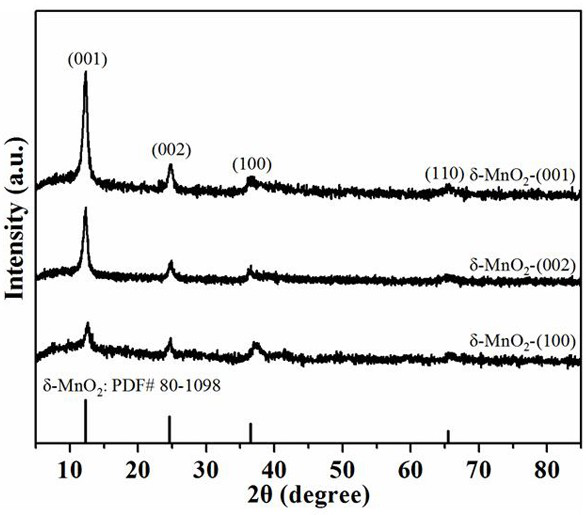
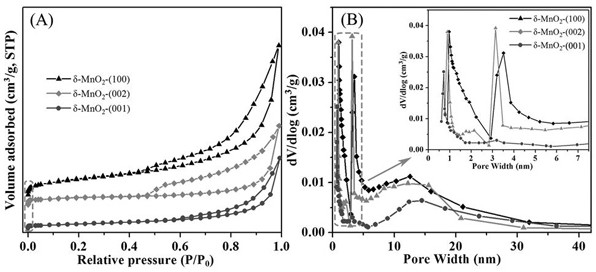
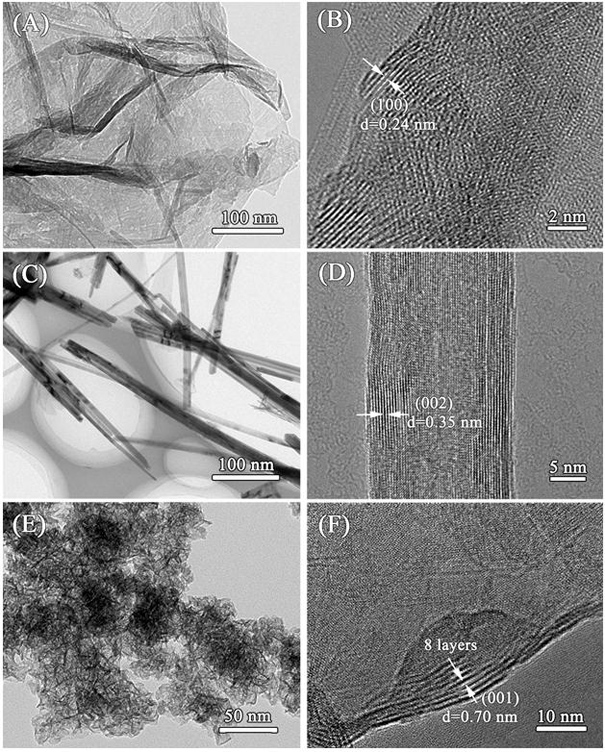
[0034] Porous MnO with high selectivity (~75%) exposed (100) facets 2 Preparation method: prepare 50 mL of KMnO with a concentration of 0.126 mol / L respectively 4 solution and 80 mL of 0.04 mol / L (NH 4 ) 2 C 2 o 4 ·H 2O solution, after mixing and stirring, add 0.3 g of cetyltrimethylammonium bromide, and stir magnetically at 60 °C for half an hour. The mixed solution was transferred to a 200mL autoclave, hydrothermally treated at 100°C for 24 h, cooled naturally at room temperature, filtered, washed with absolute ethanol and distilled water for three times, and the obtained powder was dried at 90°C for 12 h. Then, the temperature was raised to 300 °C at a rate of 5 °C / min and kept for 2 h to obtain the final product, which was named δ-MnO 2 -(100). Known through calculation, the productive rate of this catalyst is about 95%.
Embodiment 2
[0036] Porous MnO with high selectivity (~65%) exposed (002) facets 2 The preparation method: 10 mmol of KMnO 4 , 20mmol of (NH 4 ) 2 C 2 o 4 ·H 2 O and 0.15 g of urea were dissolved in 70 mL of distilled water and subjected to magnetic stirring for half an hour. The mixed solution was transferred to a 100 mL autoclave, hydrothermally treated at 90 °C for 24 h, cooled naturally at room temperature, filtered, washed with absolute ethanol and distilled water for three times each, and the obtained powder was placed in an air atmosphere at 100 °C Dry for 12 h, then raise the temperature to 300 °C at a rate of 3 °C / min and hold for 2 h to obtain the final product, named δ-MnO 2 -(002). Known through calculation, the productive rate of this catalyst is about 97%.
Embodiment 3
[0038] Porous MnO with high selectivity (~72%) exposed (001) facets 2 Preparation method: 0.45 g of KMnO 4 , 0.20 g (NH 4 ) 2 C 2 o 4 ·H 2 O, 0.1 g of urea and 1 mL of 37 wt% HCl were dissolved in 40 mL of distilled water. The mixed solution was transferred to a 100 mL autoclave, hydrothermally treated at 100 °C for 24 h, cooled naturally at room temperature, filtered, washed with absolute ethanol and distilled water for three times each, and the obtained powder was dried at 100 °C for 10 h , and then raised to 300 °C at a rate of 3 °C / min and held for 2 h to obtain the final product, which was named δ-MnO 2 -(001). Known through calculation, the productive rate of this catalyst is about 94%.
[0039] X-ray powder diffraction (XRD): The phase characterization of the sample was determined by the X' pert pro powder diffractometer of Panalytical Company, the detector was X'celerator, and the copper target (Cu Kα, λ = 0.154 nm) was used as the excitation ray source. The w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com