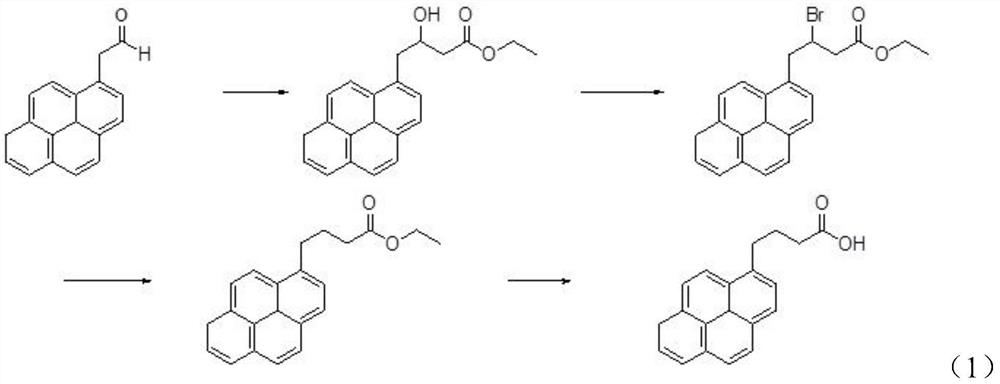
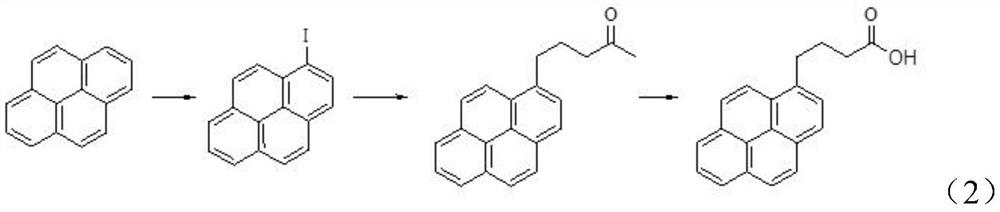
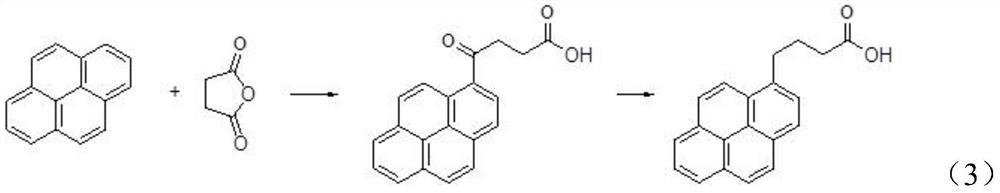
Preparation method of 1-pyrene butyric acid
A technology of pyrene butyric acid and pyrene butyrate, which is applied in the field of preparation of 1-pyrene butyric acid, can solve the problems of difficult operation, solvent nitrobenzene toxicity, unsuitability for industrial production, etc., achieve simple purification process and reduce purification difficulty , the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of 1-pyrene butyric acid according to the embodiment of the present invention comprises the following steps:
[0038] Step S1, F-C acylation of pyrene with chloroformyl butyrate compounds to obtain intermediate 4-oxo-4-pyrene butyrate.
[0039] That is to say, instead of succinic anhydride in the prior art, the preparation method of the present invention uses chloroformyl butyrate compounds to undergo F-C acylation reaction with pyrene to obtain the intermediate 4-oxo-4-pyrene Ester.
[0040] Specifically, the reaction is shown in the following formula (4):
[0041]
[0042] In the above reaction formula (4), (I) is the structural formula of pyrene, (II) is the structural formula of the chloroformyl butyrate compound used in the present invention, and (III) is obtained after the F-C acylation reaction in the present invention The structural formula of the intermediate 4-oxo-4-pyrene butyrate.
[0043] Wherein, R may be methyl, ethyl, propyl,...
Embodiment 1
[0062] (a) Synthesis of 4-oxo-4-pyrene butyric acid methyl ester
[0063] Add methyl chloroformylbutyrate (22.6g, 0.15mol) and methylene chloride to the reaction flask, add anhydrous aluminum chloride (40g, 0.30mol) in batches under the condition of 0°C under temperature control, and then Pyrene (30 g, 0.15 mol) was added in batches and reacted at room temperature until the pyrene was consumed. The reaction solution was poured into ice water to precipitate the product, and the filter cake was recrystallized with ethanol, filtered and dried to obtain a yellow powder (35 g, yield 73%).
[0064] The nuclear magnetic test result of product is as follows:
[0065] 1 H NMR (400MHz, DMSO): δ=2.44-2.50(m, 2H, CH 2 ),2.94-2.98(m,2H,CH 2 ),3.67(s,4H,2CH 2 ), 7.71-8.45 (m, 9H, ArH).
[0066] (b) Synthesis of 1-pyrenebutyric acid
[0067] Add 4-oxo-4-pyrene butyric acid methyl ester (31.6g, 0.1mol), diethylene glycol, hydrazine hydrate (18.9g, 0.3mol) and potassium hydroxide (17g, ...
Embodiment 2
[0071] (a) Synthesis of ethyl 4-oxo-4-pyrene butyrate
[0072] Add ethyl chloroformylbutyrate (24.7g, 0.15mol) and ethylene dichloride in the reaction flask, control the temperature and add anhydrous aluminum trichloride (40g, 0.30mol) in batches under the condition of 0°C, and then Pyrene (30 g, 0.15 mol) was added in batches and reacted at room temperature until the pyrene was consumed. The reaction solution was poured into ice water to precipitate the product, and the filter cake was recrystallized with ethanol, filtered and dried to obtain a yellow powder (34.7 g, yield 70%).
[0073] The nuclear magnetic test result of product is as follows:
[0074] 1 H NMR (400MHz, DMSO): δ=1.29-1.33(t,3H,CH 3 ),2.44-2.50(m,2H,CH 2 ),2.94-2.98(m,2H,CH 2 ),4.10-4.14(q,2H,CH 2 ), 7.71-8.45 (m, 9H, ArH).
[0075] (b) Synthesis of 1-pyrenebutyric acid
[0076] Add ethyl 4-oxo-4-pyrenebutyrate (33.1 g, 0.1 mol), diethylene glycol, hydrazine hydrate (18.9 g, 0.3 mol) and potassium hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com