Bimetal modified catalyst as well as preparation method and application thereof
A bimetallic and catalyst technology, applied in the field of catalysis, can solve the problems of reduced catalyst activity, reduced catalyst high-temperature hydrothermal stability, and low activity, and achieve the effects of improving low-temperature water resistance, increasing post-treatment difficulty, and high N2 selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
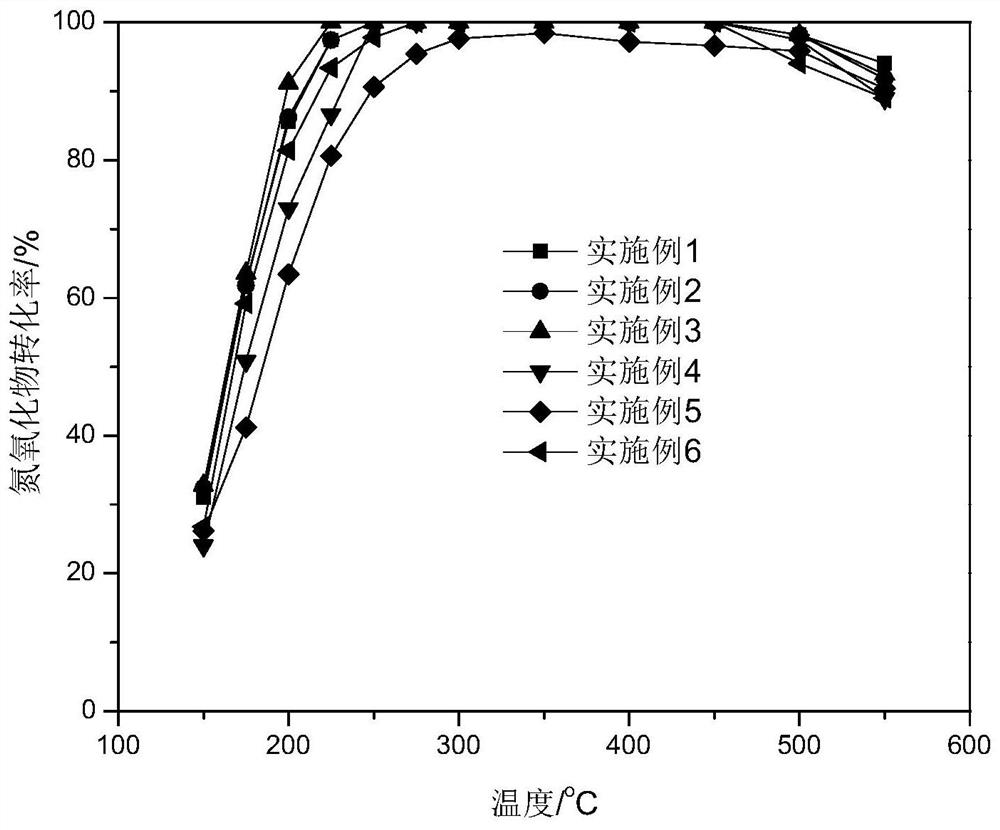
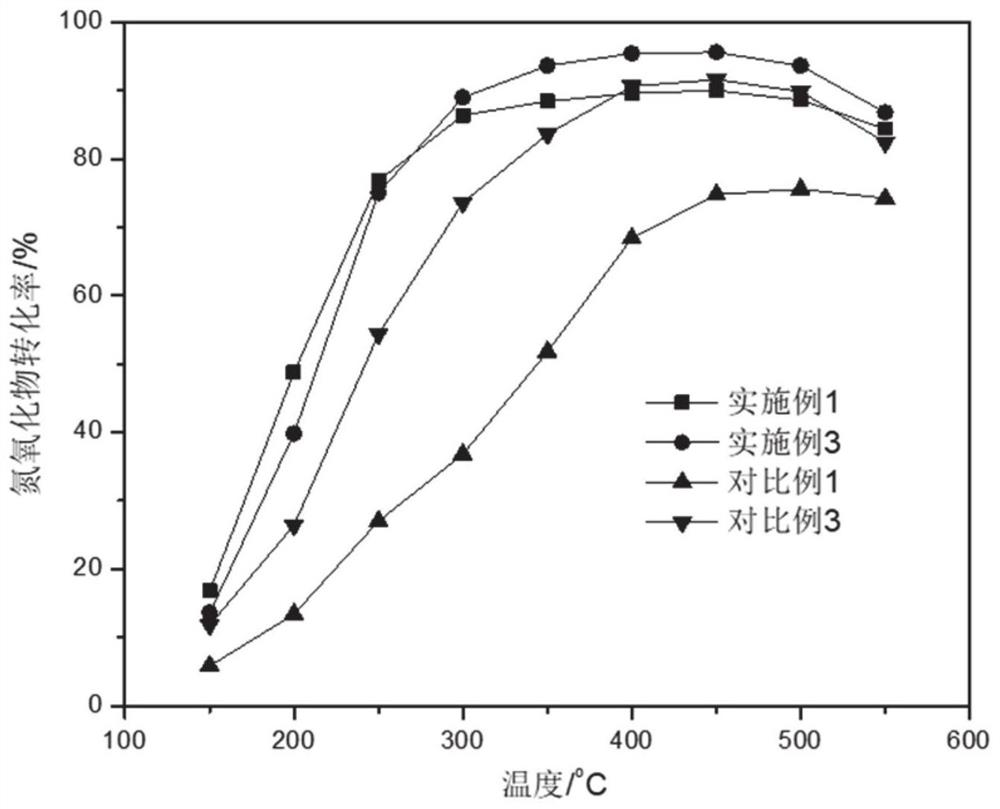
Examples
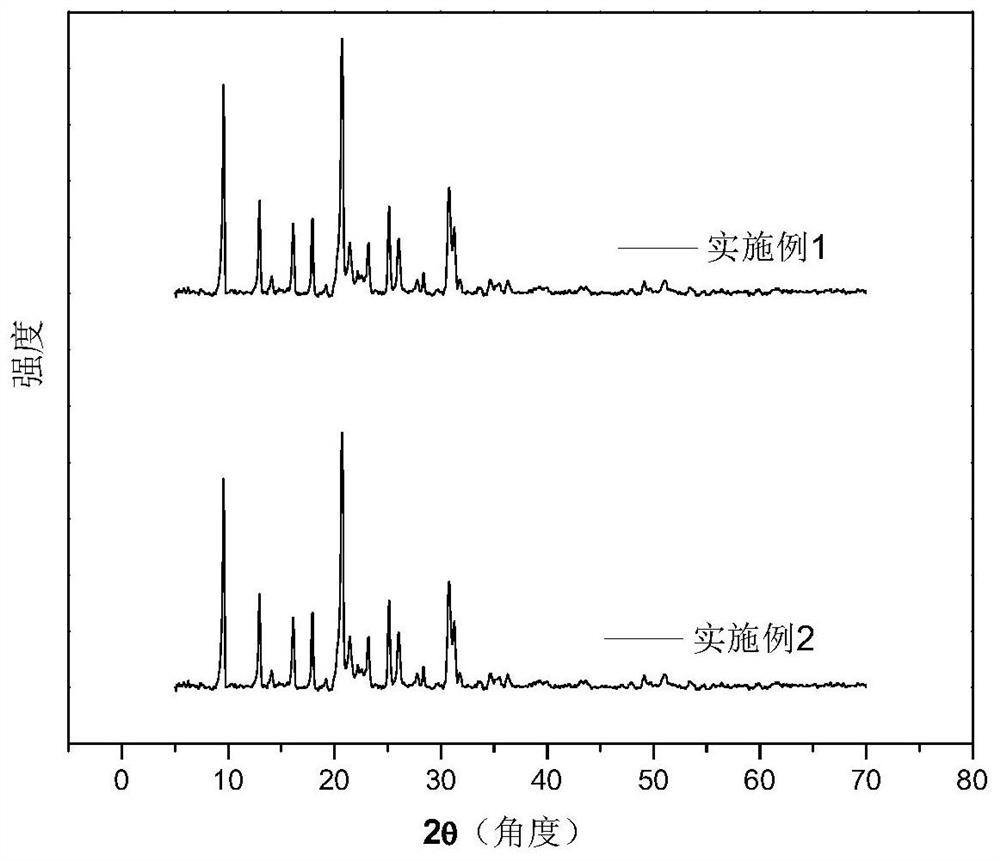
Embodiment 1
[0044] Weigh 1.01gCu(NO 3 ) 2 ·3H 2 O and 1.34gZn(NO 3 ) 2 ·6H 2 O is placed in a beaker, 15ml of deionized water is added to dissolve it, and 2.4g of tetraethylenepentamine (TEPA) is added under stirring to obtain a dark blue Cu-Zn-TEPA mixed solution A1, wherein the mol ratio of copper to zinc is 1:1.
[0045] Weigh 8.3g (the mass fraction of phosphoric acid is 85%) of phosphoric acid and place it in a beaker, add 35mL of water and stir, add 6.18g of pseudo-boehmite under heating at 80°C and continue stirring for 2 hours, then add 4.545g of triethyl Amine, 18.9g tetraethylammonium hydroxide aqueous solution (the mass fraction of tetraethylammonium hydroxide is 35%), 1.64g fumed silica obtain initial sol B1, and the silicon-aluminum atomic ratio in the initial gel wherein is 0.3;
[0046] Add the Cu-Zn-TEPA solution A1 into the initial sol B1, continue heating and stirring at 80°C for 2 hours, then cool down to room temperature and stir for 15 hours to obtain the initia...
Embodiment 2
[0049] Weigh 1.01gCu(NO 3 ) 2 ·3H 2 O and 1.34gZn(NO 3 ) 2 ·6H 2 O is placed in a beaker, 15ml of deionized water is added to dissolve it, and 1.7g of tetraethylenepentamine (TEPA) is added under stirring conditions to obtain a dark blue Cu-Zn-TEPA mixed solution A2, wherein the molar ratio of copper to zinc is 1:1.
[0050] The initial sol used in this example is B1;
[0051] Add the Cu-Zn-TEPA solution A2 to the initial sol B1, continue heating and stirring at 80° C. for 2 hours, then cool down to room temperature and stir for 15 hours to obtain the initial mixed gel C2.
[0052] The gel C2 was transferred to a reaction kettle with a polytetrafluoroethylene liner, and the temperature of the reaction kettle was raised to 200° C. for 24 hours for crystallization. After the crystallization was completed, cool to room temperature, and then filter and wash the sample. Dry the washed molecular sieve filter cake in a blast drying oven at 80°C for 12 hours, then put the drie...
Embodiment 3
[0054] Weigh 1.01gCu(NO 3 ) 2 ·3H 2 O and 0.67gZn(NO 3 ) 2 ·6H 2 O is placed in a beaker, 15ml of deionized water is added to dissolve it, and 1.7g of tetraethylenepentamine (TEPA) is added under stirring conditions to obtain a dark blue Cu-Zn-TEPA mixed solution A3, wherein the molar ratio of copper to zinc is It is 2:1.
[0055] The initial sol used in this example is B1;
[0056] Add the Cu-Zn-TEPA solution A3 to the initial sol B1, continue heating and stirring at 80°C for 2 hours, then cool down to room temperature and stir for 15 hours to obtain the initial mixed gel C3.
[0057] The gel C3 was transferred to a reaction kettle with a polytetrafluoroethylene liner, and the temperature of the reaction kettle was raised to 210° C. for 24 hours for crystallization after filling the kettle. After the crystallization was completed, cool to room temperature, and then filter and wash the sample. Dry the washed molecular sieve filter cake in a blast drying oven at 80°C fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com