Preparation method of 2, 2-dimethyl cyclopentanone
A technology of dimethyl cyclopentanone and methyl cyclopentanone is applied in the field of preparation of 2,2-dimethyl cyclopentanone, and can solve the problems of low yield, difficulty in realizing industrialized production, and difficulty in controlling reaction conditions and the like , to achieve the effect of improving the total yield, easy availability of raw materials and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
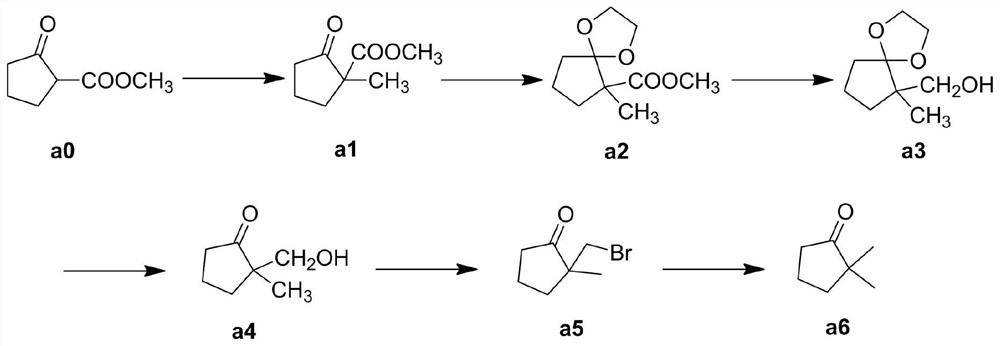
[0039] Example 1: Preparation of 2-methyl-2-methoxycarbonylcyclopentanone (a1)
[0040] Add 2-methoxycarbonylcyclopentanone (390 g, 2.75 mol) and tetrahydrofuran (2 L) into a 5 L three-neck reaction flask, and lower the temperature to 0 °C under mechanical stirring. Potassium hydroxide (168 g, 3 mol) was added in batches, stirring was continued for 30 min after the addition was complete, methyl bromide (262 g, 2.75 mol) was added dropwise to the system, and the dropwise temperature was controlled at 0°C. After the dropwise addition was completed, stirring was continued for 12 hours. Pour the reaction solution into water, add dichloromethane to extract three times, combine the organic phases, and wash once with water and saturated salt. Dry over anhydrous sodium sulfate, and remove the solvent under reduced pressure to obtain 412 g of light yellow 2-methyl-2-methoxycarbonylcyclopentanone. The product does not need further purification, and the yield is 97%.
Embodiment 2
[0041] Example 2: Preparation of 2-methyl-2-methoxycarbonylcyclopentanone vinyl ketal (a2)
[0042]Add 2-methyl-2-methoxycarbonylcyclopentanone (390 g, 2.5 mol), ethylene glycol (465 g, 7.5 mol), p-toluenesulfonic acid (43 g, 10%), and 2 L of methylcyclohexane, heated up to 100°C and stirred and refluxed for 4-5 h. After the reaction, add water to the reaction system, separate the liquids, extract the water phase with methylcyclohexane for 3 times, combine the organic phases, and wash with water. Wash with saturated saline. Dry over anhydrous sodium sulfate, and remove the solvent under reduced pressure to obtain 495 g of 2-methyl-2-methoxycarbonylcyclopentanone ethylene ketal. The product does not need further purification, and the yield is 99%.
Embodiment 3
[0043] Example 3: Preparation of 2-hydroxymethyl-2-methylcyclopentanone vinyl ketal (a3)
[0044] Add 2-methyl-2-methoxycarbonylcyclopentanone ethylene ketal (200 g, 1mol) and 2-methylbutanol (2 L) successively into a 5 L three-neck round bottom flask, raise the temperature to 75°C, and Metal sodium block (46 g, 2 mol) was cut into flakes and added to the reaction flask in batches, and the reaction was continued at this temperature for 1 h after addition. After the reaction is over, slowly add 1 L of ethanol to the system at 0°C to quench the unreacted sodium metal, slowly pour the reaction solution into water, add ethyl acetate to extract three times, combine the organic phases, wash with water, and wash with saturated salt Wash with water. Dry over anhydrous sodium sulfate, and remove the solvent under reduced pressure to obtain 160 g of 2-hydroxymethyl-2-methylcyclopentanone vinyl ketal. The product does not need further purification, and the yield is 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com