Mutant with thermal stability of gamma-glutamine methylamine synthetase and encoding gene, amino acid sequence and application of gamma-glutamine methylamine synthetase mutant
A glutamine methylamine and gene sequence technology, applied in the field of mutants of γ-glutamine methylamine synthetase, can solve problems such as easy inactivation and hindering synthetase, so as to reduce reaction cost, release ATP inhibition, and reduce production cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Construction of embodiment 1 mutant
[0045] The theanine synthase gene gmas sequence derived from Methylovorus mays, codon-optimized sequence is SEQ ID NO.1, connected into plasmid pET28(a), and transformed into E.coliBL21 to obtain the original strain E. .coliBL21-pET28(a)-gmas named WT.
[0046] The method for obtaining gmas DBM of gamma-glutamine methylamine synthetase mutant, comprises the steps:
[0047] 1. Follow the QuickMutation TM Gene random mutation kit design primers and random mutation, the primer sequence is:
[0048] random-F: GATATACATATGAAGAGCCTGGAAG; SEQ ID NO.4;
[0049] random-R: cccaagcttGTAGAATTGAACATAGCGG; SEQ ID NO.5;
[0050] 2. Random mutation PCR reaction
[0051] The random mutation PCR reaction refers to the following table to set up the random mutation PCR reaction system:
[0052] Table 1
[0053] Reagent final concentration volume Double distilled water or MilliQ water - 12.2μL RandomMut buffer(10×) 1× ...
Embodiment 2
[0063] Theanine was prepared by biotransformation using mutant gmas DBM wet bacteria as biocatalyst and sodium glutamate and ethylamine hydrochloride as substrates. The catalytic system is 10L, and the catalytic system comprises 400mM sodium glutamate, 440mM ethylamine, 160mM magnesium chloride, 2mM ATP, 160mM sodium hexametaphosphate and 60g / L gmas DBM wet thallus and 40g / LPPK wet thallus. The reaction system was reacted at 45°C in an aqueous solution with pH=7. After the reaction, the cells were removed by centrifugation, and the samples were filtered through a 0.22 μm membrane to detect the production of theanine by HPLC. The maximum output of theanine is 345mM, which is 60.1g / L, the reaction time is 16 hours, and the production intensity is 3.76g / L / h, which is 2.0 times that of the original bacteria.
Embodiment 3
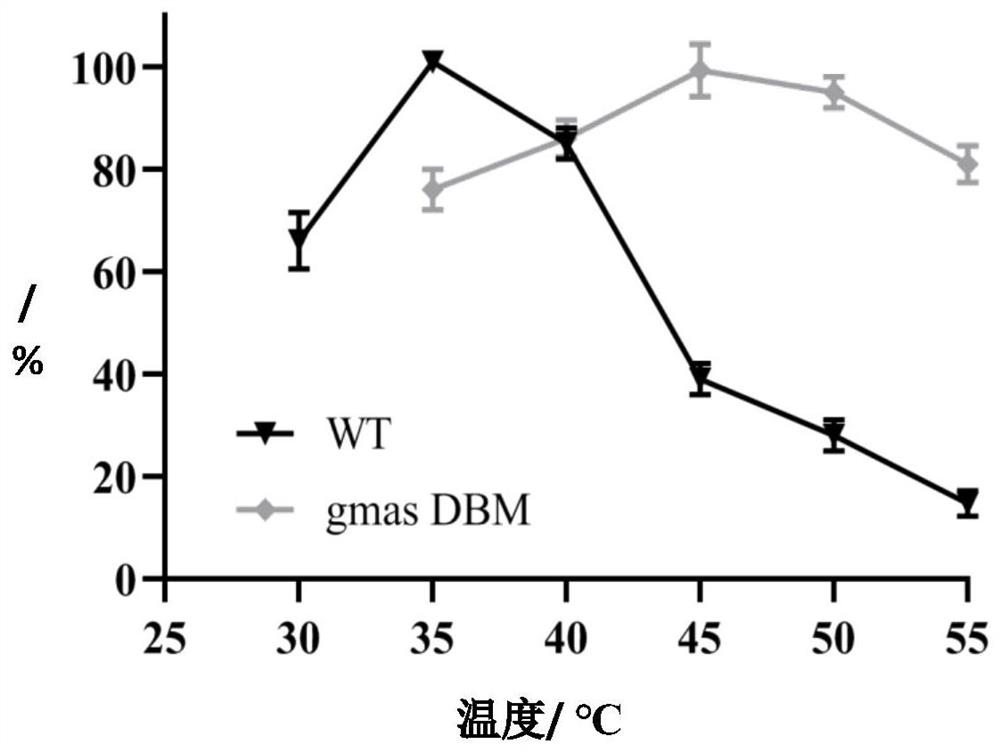
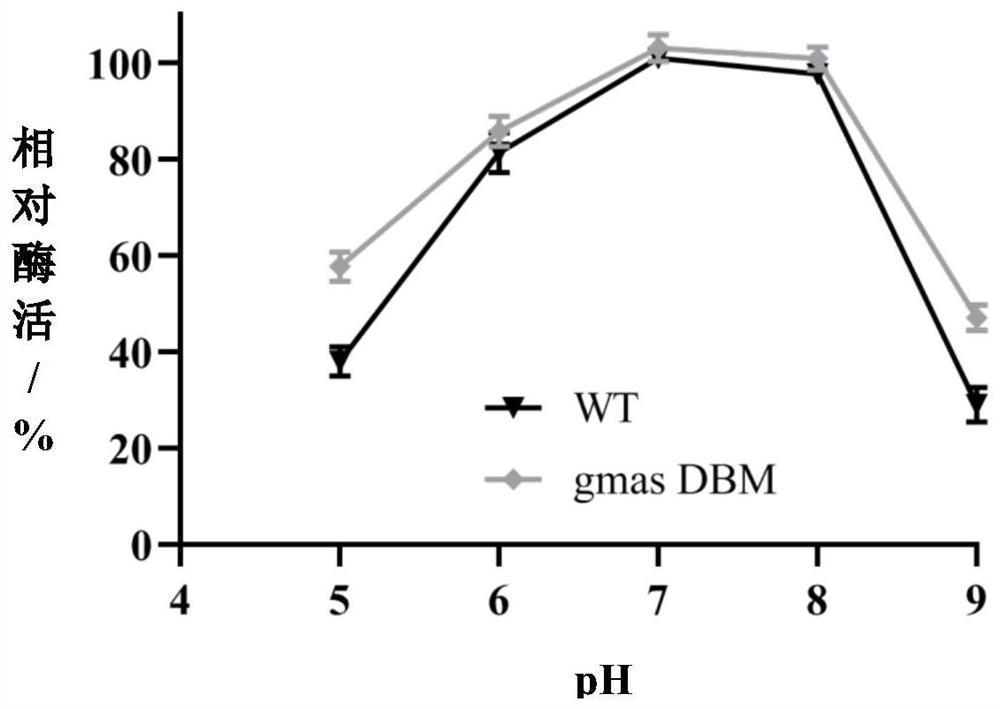
[0064] The influence of embodiment 3 temperature on enzyme property
[0065] Temperature can change the catalytic reaction speed of the enzyme, and can also lead to the reduction or inactivation of the enzyme protein activity. Simultaneously determine the optimal reaction temperature of unmutated WT and mutant gmas DBM. Respectively placed at 30, 35, 40, 45, 50 ° C for 60 minutes. In a PBS solution with pH=7, the reaction system contained 50 mM sodium glutamate, 55 mM ethylamine, 40 mM magnesium chloride and 30 mM ATP and 15 g / L gmas cells. The change of theanine in the reaction solution was regularly measured by HPLC, and the enzyme activity value of WT measured at 35°C was taken as 100%, and the relative enzyme activity at other temperatures was calculated. Experimental results such as figure 1 It was shown that the optimal temperature of WT was 35°C, while that of gmasDBM was 45-50°C.
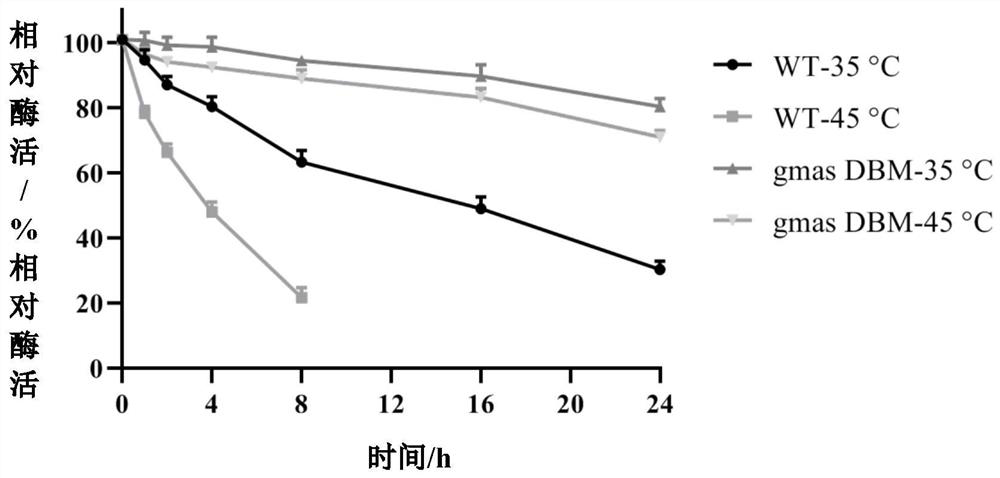
[0066] The non-mutated WT and mutant gmas DBM were incubated at 35 and 45°C, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com