Green preparation method of menadione sodium bisulfite
A technology of menadione sodium bisulfite and sodium bisulfite, which is applied in the field of green preparation of menadione sodium bisulfite, can solve the problem of high prices of 2-methylnaphthalene and m-chloroperoxybenzoic acid, and sulfurous acid Menadione hydrogen sodium has poor reaction selectivity and no industrial application value, etc., and achieves the effects of easy scale-up and control, less side reactions, high specificity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
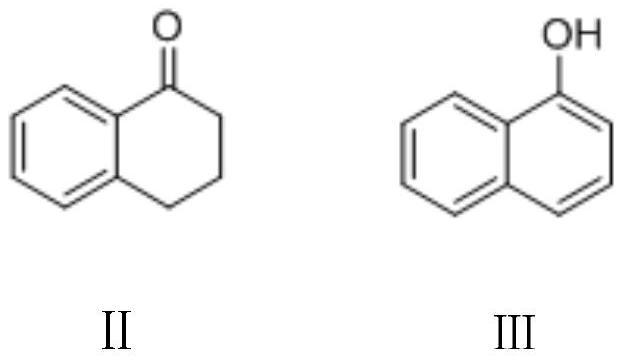
[0064] Embodiment 1: the preparation of 1-naphthol (Ⅲ)
[0065] To a 500-milliliter four-neck flask connected with a stirring, thermometer, reflux condenser, air duct and 30 wt% sodium hydroxide aqueous solution absorption device, add 300 grams of 1,2-dichloroethane, 73.0 grams (0.5 moles) of 3, 4-Dihydro-1(2H)-naphthalenone (II), heat, keep at 40-45°C, slowly pass in 39.0 g (0.55 moles) of chlorine gas, and pass in about 2-3 hours, then 45- Stir and react at 50°C for 3 hours, cool to 20-25°C, add 60.0 g (0.6 moles) of 40wt% sodium hydroxide aqueous solution, stir and react at 30-35°C for 2 hours, cool to 20-25°C, acidify the pH of the system with 30wt% hydrochloric acid The value is 3.0-4.0, separate layers, the water layer is extracted 3 times with 1,2-dichloroethane, 50 grams each time, the organic phases are combined, the solvent is recovered by distillation, and dried to obtain 69.5 grams of 1-naphthol (Ⅲ), Yield 96.5%, gas phase purity 99.8%.
Embodiment 2
[0066] Embodiment 2: the preparation of 1-naphthol (Ⅲ)
[0067] To a 500-milliliter four-neck flask connected with a stirring, thermometer, reflux condenser, air duct and 30 wt% sodium hydroxide aqueous solution absorption device, add 300 grams of 1,2-dichloroethane, 73.0 grams (0.5 moles) of 3, 4-Dihydro-1(2H)-naphthalenone (II), 62.5g (0.6mol) 35wt% hydrochloric acid, add 61.5g (0.55mol) 30wt% hydrogen peroxide dropwise at 35-40°C for about 3-4 hours After the dropwise addition is completed, stir and react at 40-45°C for 4 hours, cool to 20-25°C, add 60.0 g (0.6 moles) of 40wt% sodium hydroxide aqueous solution, stir and react at 30-35°C for 2 hours, and cool to 20-25°C , 30wt% hydrochloric acid acidification system pH value is 3.0-4.0, layering, the aqueous layer is extracted 3 times with 1,2-dichloroethane, 50 grams each time, the organic phase is combined, the solvent is recovered by distillation, and dried to obtain 69.1 grams of 1 -Naphthol (Ⅲ), yield 96.0%, gas phase ...
Embodiment 3
[0068] Embodiment 3: the preparation of 1-naphthol (Ⅲ)
[0069] To a 500-milliliter four-neck flask connected with a stirring, thermometer, reflux condenser, air duct and 30 wt% sodium hydroxide aqueous solution absorption device, add 300 grams of dichloromethane, 73.0 grams (0.5 moles) of 3,4-dihydro- 1(2H)-Naphthone (II), 111g (0.55mol) 40wt% hydrobromic acid, add 56.0g (0.5mol) 30wt% hydrogen peroxide dropwise between 25-30°C, and dropwise add in about 3-4 hours , after this 25-30 ℃ stirring reaction 2 hours; Add 60.0 grams (0.6 mole) 40wt% sodium hydroxide aqueous solution, 25-30 ℃ stirring reaction 2 hours; 30wt% hydrochloric acid acidification system pH value is 3.0-4.0, layering, water The layer was extracted 3 times with dichloromethane, 50 g each time, the organic phases were combined, the solvent was recovered by distillation, and dried to obtain 71.0 g of 1-naphthol (III), with a yield of 98.6% and a gas phase purity of 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com