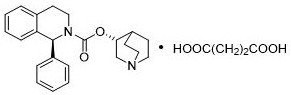
Synthesis process of solifenacin succinate
A technology of solifenacin succinate and a synthesis process, applied in the field of medicine, can solve the problems of unstable tetrahydroisoquinoline carbamoyl chloride, complicated operation of solifenacin, and low conversion rate of transesterification, and the like. The effect of reducing reversible reactions, simple operation, and reducing operating procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
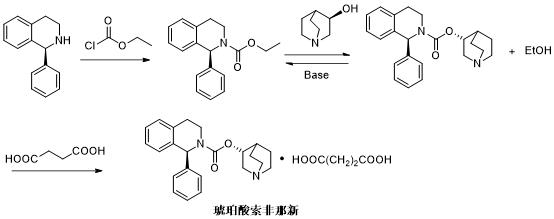
[0027] see Figure 4 As shown, the technical solution adopted in this embodiment is:
[0028] 1. First add 10.0g (R)-3-quinuclidinol to 800.0ml acetonitrile, after it dissolves, add 20.0g diphosgene dropwise to the above system in an ice-water bath, sprinkle Remove from the ice-water bath, react at room temperature for 16 hours, and concentrate to obtain 13.4 g of chloroformic acid-(R)-3-quinuclidinyl ester.
[0029] 2. Add 11.3g (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline and 8.2g triethylamine to 100.0ml dichloromethane, add dropwise to the 13.4g of chloroformic acid-(R)-3-quinuclidinine solution diluted with 60.0ml of dichloromethane, stirred for 3~10min after adding, removed the ice-water bath, continued to stir the reaction, and concentrated solifenacin crude product, Dissolve with 100.0 ml of dichloromethane, wash the organic phase with water, and concentrate to obtain 17.6 g of abdozofenacin.
[0030] 3. Add 17.6g of solifenacin oil to 20.0mL of ethyl acetate, then a...
Embodiment 2
[0032] see Figure 5 As shown, the technical solution adopted in this embodiment is:
[0033] 1. First add 10.0g (R)-3-quinuclidinol to 600.0ml THF, after it dissolves, add 20.0g diphosgene dropwise to the above system in an ice-water bath, sprinkle it after the dropwise addition In an ice-water bath, react at room temperature for 16 hours, and concentrate to obtain 13.8 g of chloroformic acid-(R)-3-quinuclidinyl ester.
[0034] 2. Add 11.3g (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline and 6.4g pyridine to 100.0ml dichloromethane, add dropwise with 62.0ml 13.8g of chloroformic acid-(R)-3-quinuclidinine solution diluted with dichloromethane, stirred for 3~10min after adding, removed the ice-water bath, continued to stir the reaction, concentrated solifenacin crude product, and then used 100.0ml of dichloromethane was dissolved, the organic phase was washed with water, and concentrated to obtain 18.0g of solifenacin.
[0035] 3. Add 18.0g of solifenacin oil to 23.0mL of ethyl ...
Embodiment 3
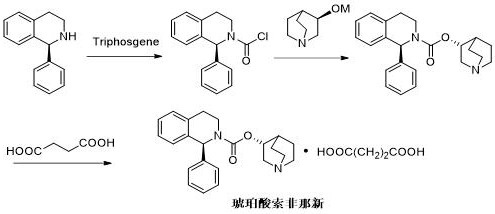
[0038] see Figure 4As shown, the difference from Example 1 is: replace the reaction solvent therein for experiments, and change one of the solvent components each time.
[0039] 1 (R)-3-quinuclidinol is added to the solvent and diphosgene to generate the reaction solvent of chloroformic acid-(R)-3-quinuclidinate respectively using acetonitrile, tetrahydrofuran, dioxane, dichloro Methane, the chloroformic acid-(R)-3-quinuclidin ester that obtains, the total yield that final preparation obtains solifenacin succinate is respectively acetonitrile (63%), tetrahydrofuran (61%), dioxane ( 47%), dichloromethane (29%).
[0040] 2 (R)-3-quinuclidinol is dissolved in acetonitrile and reacts with phosgene, diphosgene or triphosgene respectively to generate chloroformate-(R)-3-quinuclidinate, and finally prepares solifena succinate The new total yields are all about 60%.
[0041] 3 Use morpholine, N- Methylmorpholine, triethylamine, pyridine, 4-picoline are used as base, and tetrahydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com