Double-ratio type hemicyanine dye molecule as well as synthesis method and application thereof
A synthetic method and ratio-based technology, applied in the field of double-ratio semicyanine dye molecules and their synthesis, can solve the problems of inability to provide signals for distinguishing hypoxic levels, insufficient robustness of molecular imaging, false positives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
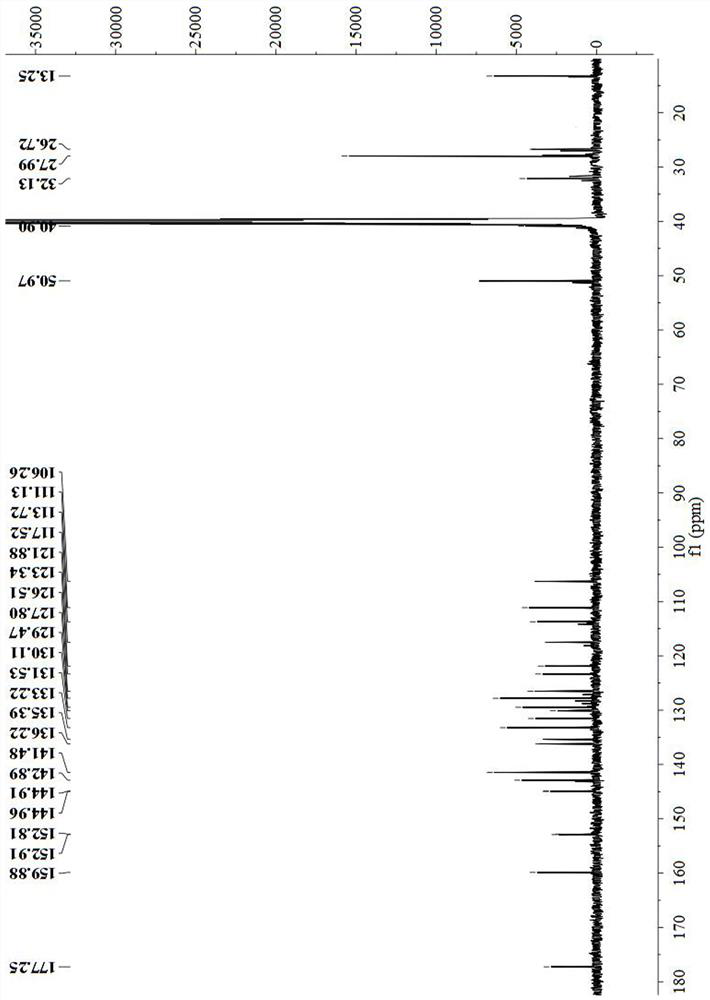
Image
Examples
Embodiment 1
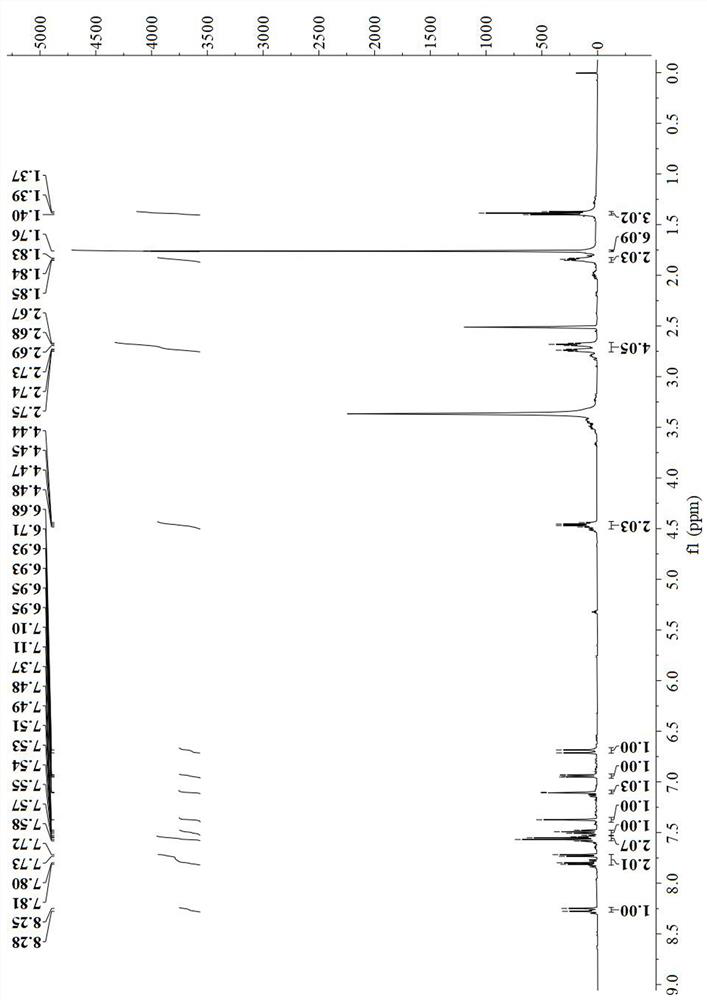
[0053] Synthesis and Characterization of Dye Molecule AS-Cy-1
[0054] Dissolve 255.5mg (0.5mmol) of raw material 1b in N,N-dimethylformamide (DMF), add 63.1mg (0.5mmol) of resorcinol and 12mg of hydrogenated sodium in a round-bottomed flask, under nitrogen protection and Stirring at 40°C for 8 hours;
[0055] After the reaction was completed, the reaction solution was poured into ice water and mixed, and 0.5ml of perchloric acid was added to allow the solid to be precipitated, and the crude product was obtained by suction filtration;
[0056] The obtained crude product was purified with a silica gel (200-300 mesh) chromatographic column with a volume ratio of 50:1 of dichloromethane and methanol as an eluent to obtain 106.3 mg of a blue solid (yield rate: 51.3 %).
[0057] refer to figure 1 , 1 H NMR (500MHz, DMSO-d 6 )δ8.26(d, J=14.4Hz, 1H), 7.77(dd, J=39.7, 7.7Hz, 2H), 7.58–7.53(m, 2H), 7.49(t, J=7.4Hz, 1H), 7.37(s,1H),7.11(d,J=2.3Hz,1H),6.94(dd,J=8.5,2.3Hz,1H),6.70(d,J...
Embodiment 2
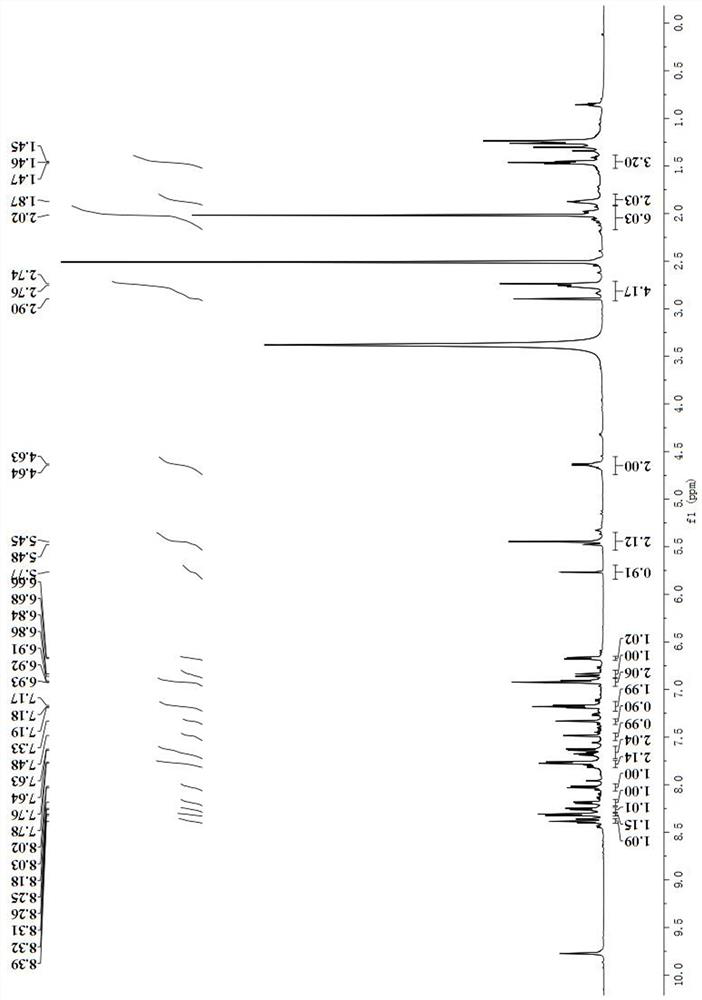
[0061] Dye molecule BS-Cy-NO 2 Synthesis and characterization of
[0062] Dissolve 282.1mg (0.5mmol) of BS-Cy-1 in N,N-dimethylformamide (DMF) and add 108mg (0.5mmol) of brominated p-nitrotoluene and 17.3mg of potassium carbonate in a round bottom flask , stirred and reacted for 4 hours under nitrogen protection and 60°C;
[0063] After the reaction was completed, the reaction solution was poured into ice water and mixed, and 0.5ml of perchloric acid was added to allow the solid to be precipitated, and the crude product was obtained by suction filtration;
[0064] The obtained crude product was purified with a silica gel (200-300 mesh) chromatographic column with a volume ratio of 60:1 of dichloromethane and methanol as an eluent to obtain 227.6 mg of a blue solid (yield rate: 65.1 %).
[0065] refer to image 3 , 1 H NMR (600MHz, DMSO-d 6 ))δ8.38(t, J=11.8Hz, 1H), 8.32(d, J=8.6Hz, 1H), 8.26(d, J=8.9Hz, 1H), 8.19(d, J=8.2Hz, 1H ), 8.02(d, J=8.9Hz, 1H), 7.77(d, J=8.6Hz, ...
Embodiment 3
[0069] Dye molecule BS-Cy-NO 2 Spectrum in response to nitroreductase:
[0070] The dye molecule BS-Cy-NO prepared in configuration embodiment 2 2 DMSO mother solution with a concentration of 10mM; then add 1998μL liquid (PBS:methanol=8:2) and 2μL probe mother solution to the UV and fluorescent dishes respectively, add nitroreductase and NADH to the dish, and the ultraviolet spectrum can be observed The peak falls at 670nm and rises at 750nm, and the peak of the fluorescence spectrum falls at 760nm and rises at 800nm, showing a double ratio change, such as Figure 5 , Image 6 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com