Preparation method of etimicin sulfate effervescent tablet
A technology for etimicin sulfate and effervescent tablets, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve problems such as substandard hardness of tablets, and achieve rapid absorption. , the convenience of taking, the effect of increasing medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
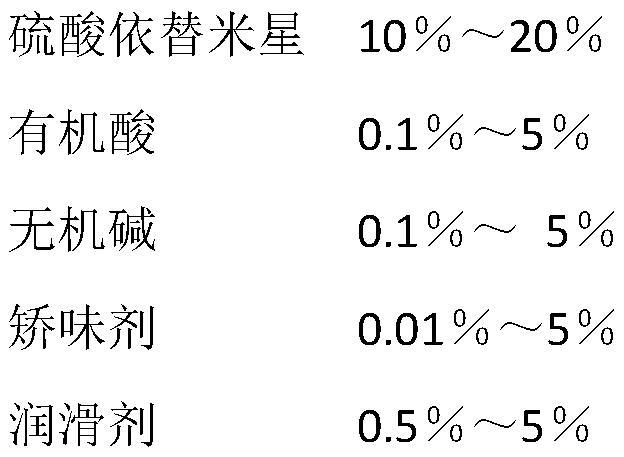
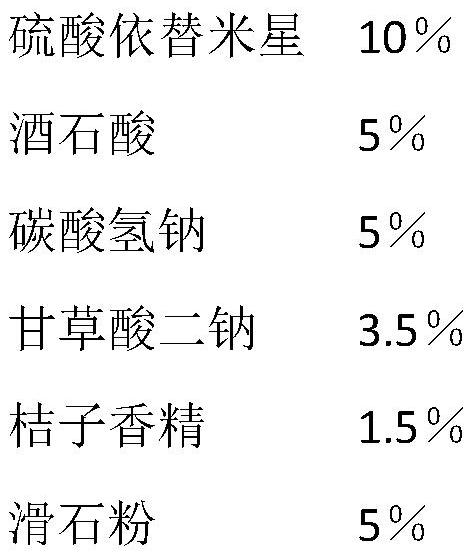
[0040] An etimicin sulfate effervescent tablet, its main components include 10% etimicin sulfate, 5% tartaric acid, 5% sodium bicarbonate, 3.5% disodium glycyrrhizinate, 1.5% orange flavor, Talc 5%, the rest anhydrous lactose.
[0041] Preparation:
[0042] Step 1. Etimicin sulfate fine powder and 1 / 3 anhydrous lactose are mixed uniformly in equal increments;
[0043] Step 2. Mix tartaric acid, sodium bicarbonate, disodium glycyrrhizinate, orange essence, talcum powder and 2 / 3 anhydrous lactose in equal increments and pass through a 200-mesh sieve;
[0044] Step 3. After mixing the mixture of Step 1 and Step 2 uniformly by the method of equal increments, the tablet can be directly compressed. The friability of the formula is 0.2%, which ensures the impact resistance of the tablet in the later transportation process, the disintegration time limit is within 2 minutes, and the taste is better, which is obviously better than other examples.
Embodiment 2
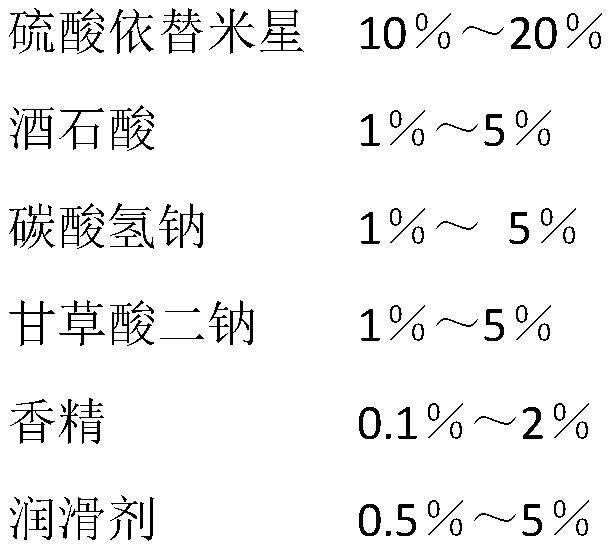
[0046] An etimicin sulfate effervescent tablet, its main components include 20% etimicin sulfate, 5% citric acid, 5% sodium carbonate, 0.3% aspartame, and 0.7% glycyrrhizin in weight percent , peach essence 2.5%, micronized silica gel 1%, and the rest is anhydrous lactose.
[0047] Preparation:
[0048] Step 1. Etimicin sulfate powder and 1 / 3 anhydrous lactose are mixed uniformly in equal increments;
[0049] Step 2. Mix citric acid, sodium carbonate, aspartame, glycyrrhizin, peach essence, orange, micronized silica gel and 2 / 3 anhydrous lactose in equal increments and pass through a 200-mesh sieve;
[0050] Step 3. After mixing the mixture of Step 1 and Step 2 uniformly by the method of equal increments, the tablet can be directly compressed. The friability of the formula is 0.6%, which ensures the impact resistance of the tablet during later transportation, the disintegration time is within 3 minutes, and the taste is suitable.
Embodiment 3
[0052] An etimicin sulfate effervescent tablet, the main components of which include 30% etimicin sulfate, 5% tartaric acid, 5% sodium bicarbonate, 3% disodium glycyrrhizinate, 2% orange flavor, Talc 2.5%, the rest is anhydrous lactose.
[0053] The friability of the formula is 0.9%, the disintegration time limit is within 3 minutes, and the taste is suitable.
[0054] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com