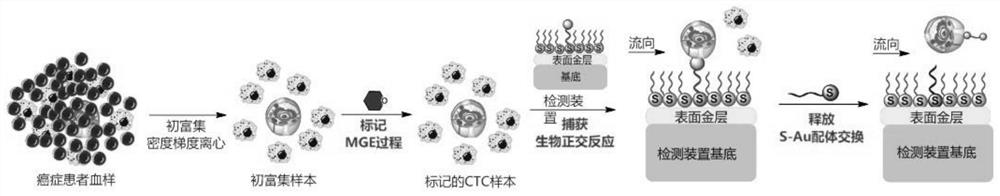
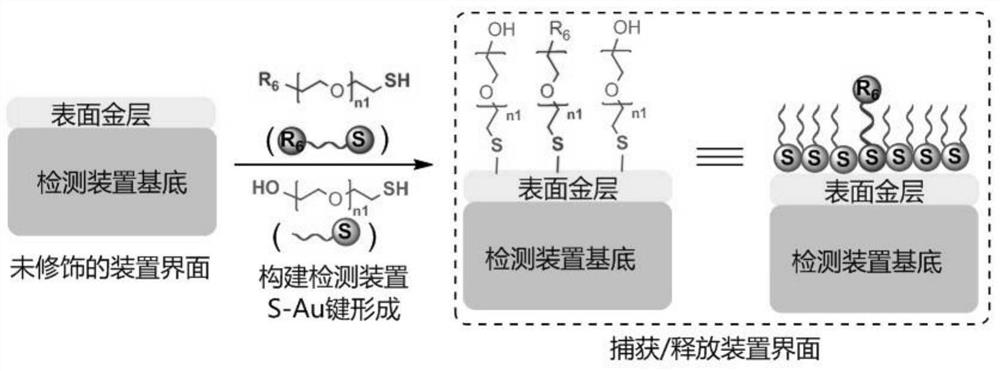
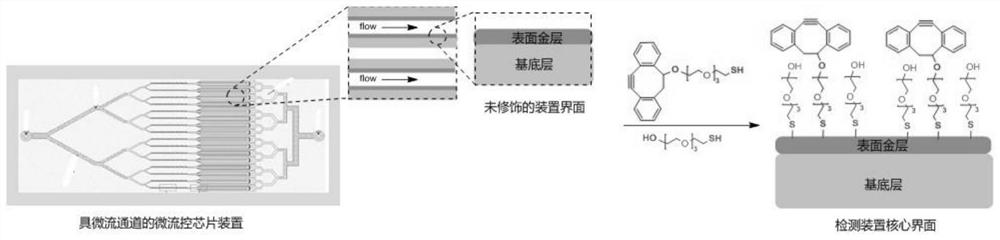
Circulating tumor cell detection method
A technology of tumor cells and detection methods, which is applied in the field of detection of circulating tumor cells based on bio-orthogonal metabolic glycoengineering labeling, can solve the problem of inability to achieve accurate detection, limited accuracy and non-destructiveness, and insufficient detection accuracy of CTCs for non-destructive capture/ Issues such as releasing CTC
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1 Preparation of labeled molecules
[0079]
[0080] Mannose amino acid hydrochloride (1.0g, 4.64mmol) was dissolved in ethanol (10mL), sodium bicarbonate (0.4g, 4.77mmol) was added, after stirring for 15min, chloroacetyl chloride (0.63g, 5.56mmol) was added, and stirred at room temperature for 1 hours, the solvent was evaporated to dryness, the residue was dissolved in N,N-dimethylformamide DMF (10mL), sodium azide (0.6g, 9.28mmol) was added, stirred overnight in an oil bath at 50°C, and the reaction solution was cooled to At room temperature, the solvent was aliquoted, the residue was dissolved in ethanol (10mL), the insoluble matter was filtered off, and the filtrate was evaporated to dryness to obtain the crude intermediate (0.91g, 74.8%), ESI-MS m / z calcd for [C 8 h 15 N 4 o 6 ] + (M+H) + :263.23; found: 263.24;
[0081] The intermediate (0.91g, 3.47mmol) was dissolved in pyridine (5mL), acetic anhydride (2mL) was added in an ice bath, transferred ...
Embodiment 2
[0082] Example 2 Preparation of labeled molecules
[0083]
[0084] h 2 SO 4 (1mL) was added dropwise to acetone (30mL) under ice bath, L-galactose (1g, 5.55mmol) was added in batches, turned to room temperature and stirred for 4 hours until completely dissolved, and saturated NaOH solution was added to adjust the solution to be neutral. The insoluble matter was filtered off, and the filtrate was concentrated to obtain an intermediate (1.4 g, 97%);
[0085] The intermediate compound (1.4g, 5.38mmol) was dissolved in dichloromethane (10mL), and TsCl (1.54g, 8.07mmol) and Et 3 N (1.09g, 10.76mmol), stirred overnight at room temperature, added methanol (1mL), the solution was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated and purified to obtain an intermediate (2.1g, 94%);
[0086] The intermediate compound (2.1g, 5.07mmol) was dissolved in DMF (10mL), sodium azide (0.66g, 10.14mmol) was added, stirred in an oil bath at 50°C overnight, the re...
Embodiment 3
[0089] Example 3. Preparation of marker molecules
[0090]
[0091] Mannose amino acid hydrochloride (1.0g, 4.64mmol) was dissolved in ethanol (10mL), sodium bicarbonate (0.4g, 4.77mmol) was added, after stirring for 15min, acetic anhydride (0.91g, 5.10mmol) was added, and stirred at room temperature for 1 hour , Evaporated the solvent to give N-acetylaminomannose intermediate crude product (0.95g, 92.6%), ESI-MS m / z calcd for [C 8 h 16 NO 6 ] + (M+H) + :222.09; found: 221.10
[0092] N-acetylaminomannose intermediate (0.95g, 4.30mmol) was dissolved in pyridine (10mL), added trityl chloride (1.8g, 6.44mmol), stirred at room temperature for 3 hours, continued to add acetic anhydride (4.38g , 42.95mmol) to react to give 2-N-acetyl-1,2,4-tri-O-acetyl-6-O-trityl-aminomannose intermediate (2.1g, 82.9%), ESI -MS m / z calcd for [C 33 h 36 NO 9 ] + (M+H) + :590.23; found: 590.23
[0093] The intermediate obtained in the previous step (2.1g, 3.56mmol) was dissolved in met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com