Synthesis method of crisaborole
A technology of crisborole and synthetic method, which is applied in the field of preparation of pharmaceutical compounds, and can solve the problems of unfavorable industrial production, heavy metal pollution, expensive raw materials or catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] According to the present invention, there is provided a synthetic method of crisborole, which uses 4-bromo-3-methylphenol as a raw material, undergoes borylation and ring closure, and then reacts with halogenated benzonitrile to obtain crisborol Ro. The method comprises the steps of:
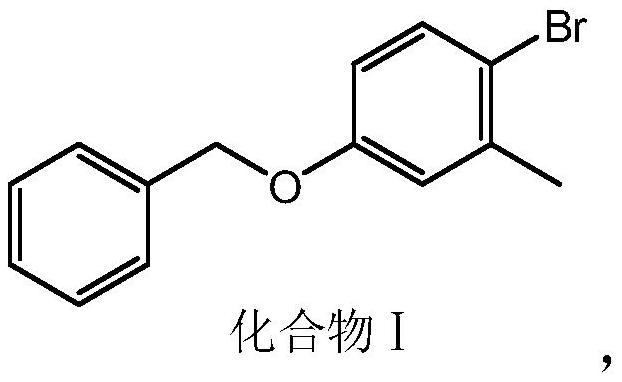
[0041] Step 1. Add 4-bromo-3-methylphenol and benzyl halide to solvent I to react to obtain compound I.
[0042]
[0043] The benzyl halide is preferably benzyl bromide or benzyl chloride.
[0044] The solvent I is selected from amide solvents, alcohol solvents, sulfone solvents, ketone solvents or nitrile solvents, preferably selected from N,N-dimethylformamide, dimethyl sulfoxide, methanol, isopropanol , acetone, butanone or acetonitrile, more preferably N,N-dimethylformamide and / or acetone.
[0045] Preferably, the reaction is carried out in the presence of an alkaline substance selected from organic or inorganic bases, preferably selected from trimethylamine, triethylamine, pota...
Embodiment 1
[0101] Add 3mol 4-bromo-3-methylphenol, 3000mL acetone, and 3.3mol potassium carbonate into the reaction kettle, add 3.6mol benzyl chloride under stirring, and reflux for 10 hours. After recovering the solvent, add 3000mL water, beat and wash, and filter Afterwards, it was dried in vacuo to obtain solid compound I with a molar yield of 96.4%.
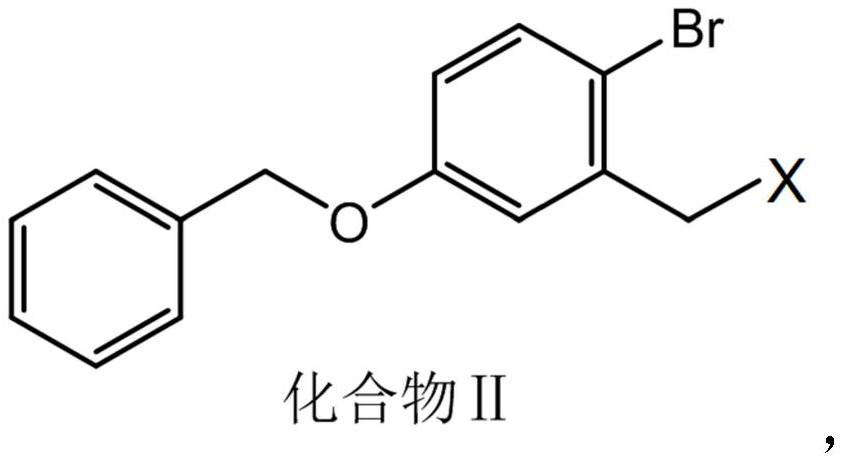
[0102] Dissolve 2 mol of the prepared compound I in 14.5 L of carbon tetrachloride, add 2 mol of NBS and 0.44 mol of BPO under stirring conditions, react at 80°C for 10 h, cool to room temperature, add 14 L of water, stir and mix, then set aside to separate layer, separate the organic phase and add anhydrous sodium sulfate to dry, remove the solvent by rotary evaporation, add ethyl acetate and petroleum ether for crystallization, the volume ratio of ethyl acetate and petroleum ether is 1:1, and compound II (wherein X is bromine ), the molar yield is 86.9%.
Embodiment 2
[0104] Take 1.5 mol of compound II prepared in Example 1 and add it into 3 L of N,N-dimethylformamide to dissolve, add 8 mol of sodium acetate under stirring condition, and stir at 80° C. for 3 h. After the reaction, cool to room temperature, add 12.5L deionized water to the reaction solution, stir, add ethyl acetate for extraction, separate the organic phase, add anhydrous sodium sulfate to dry, filter, and evaporate to remove the solvent to obtain compound III , the molar yield was 90.9%.
[0105] Take 1.2 mol of the prepared compound III, 2.4 mol of double-linked pinacol borate, and 3.6 mol of potassium acetate, and add them to 6 L of 1,4-dioxane, and then add 60 mmol of [1,1'-bis( Diphenylphosphino)ferrocene]palladium dichloride (Pd(dppf)Cl 2 ), under the protection of nitrogen, reacted at 105° C. for 6 h, heated and concentrated the reaction solution, added deionized water to stir, then stood still, and suction filtered to obtain a filter cake. Add the filter cake into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com