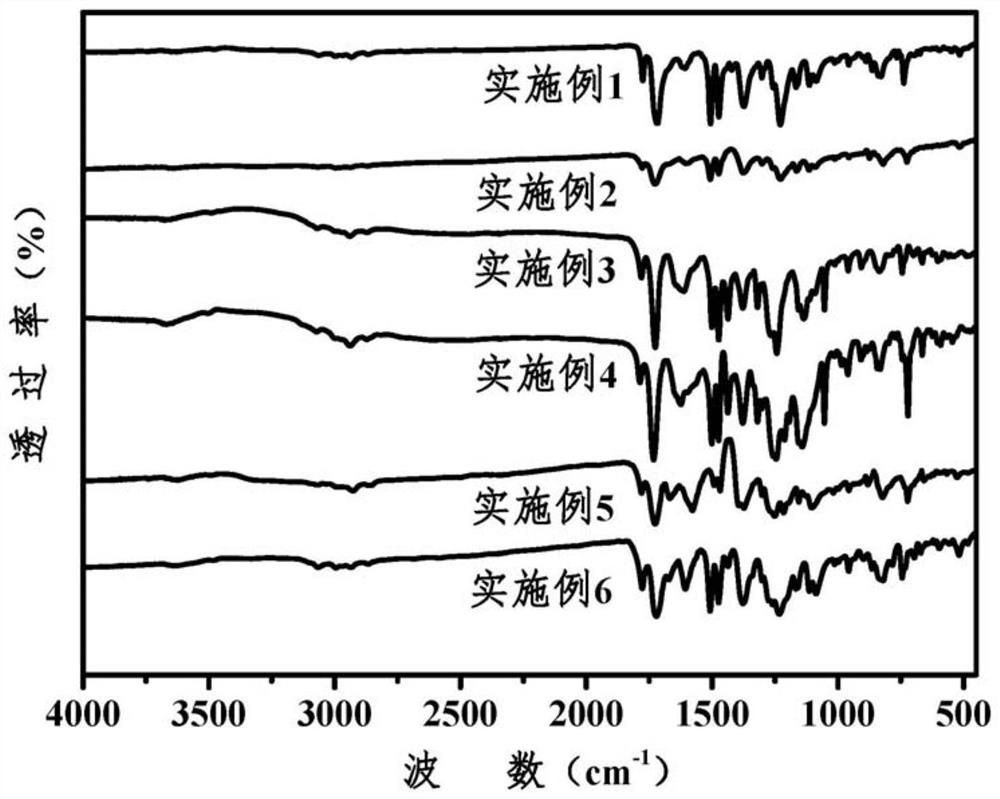
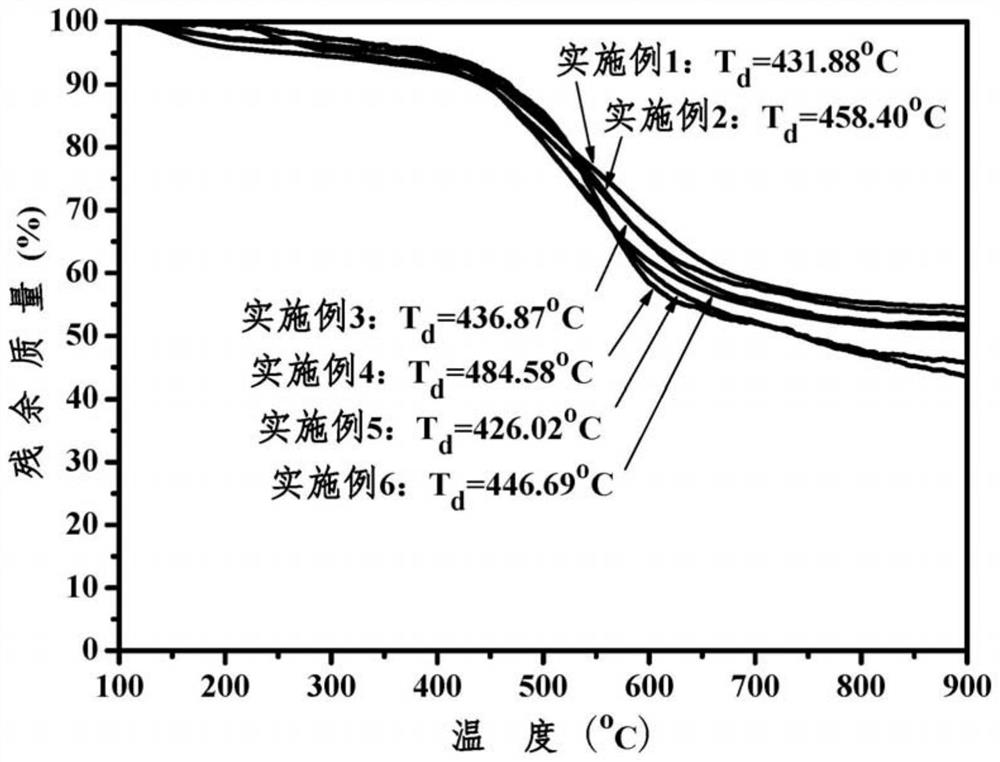
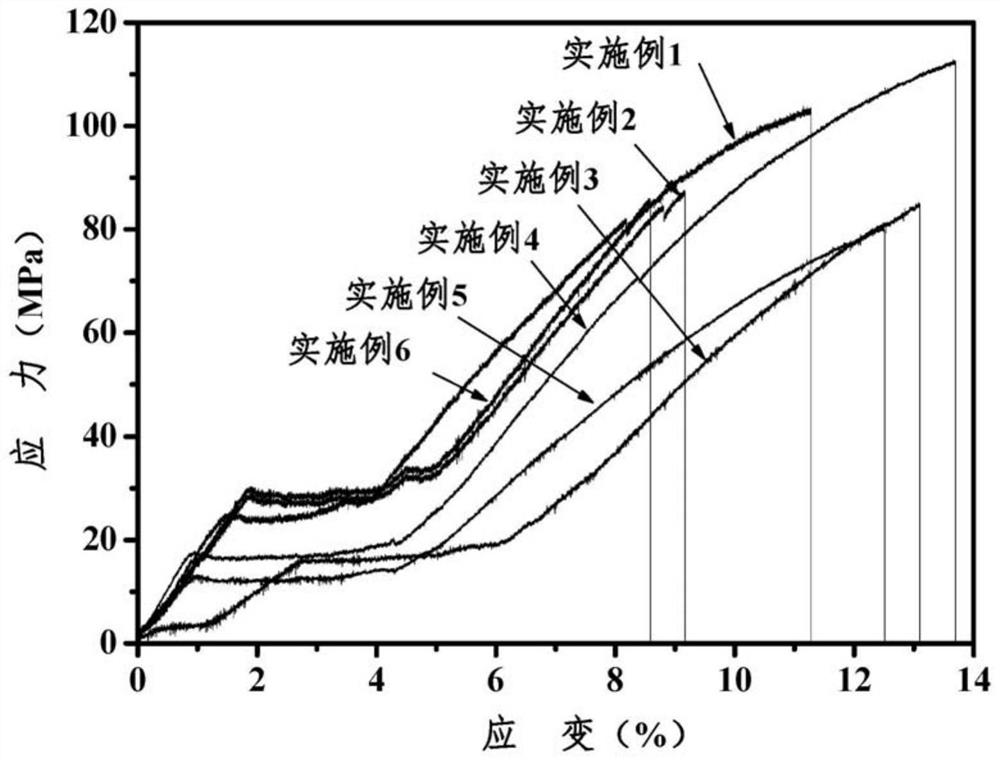
Aromatic polyimide with main chain containing benzo norbornene structure and preparation method
A technology of benzonorbornene and polyimide, which is applied in the field of polymer materials and preparation, and can solve the problems that the glass transition temperature of aromatic polyimide needs to be improved, it is not suitable for industrial production, and the number of modification methods is increased. , to achieve the effects of reducing close packing and crystallization tendency, increasing glass transition temperature, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] (1) Preparation of diketone compound.
[0059] Under the conditions of nitrogen, ice bath and mechanical stirring, take 21.62g (200mmol) of benzoquinone compound and 300mL methanol in a 500mL three-necked flask, dissolve 13.5mL (200mmol) cyclopentadiene compound in 50mL methanol in a constant pressure dropping funnel , under vigorous mechanical stirring, the methanol solution of cyclopentadiene was added dropwise to the methanol solution of 1,4-benzoquinone, kept for 1.5 hours, slowly raised to room temperature (25°C), and reacted at room temperature for 6 hours to obtain Tan solution. Methanol was removed by a rotary evaporator, and the remaining tan solid was recrystallized with n-hexane, and 32.56 g of a yellow needle-like crystal product, namely norbornenecyclohexenedione compound, was precipitated.
[0060] (2) Preparation of dinitro compounds containing benzonorbornene structure.
[0061] Under the conditions of nitrogen and magnetic stirring, take 1.74g (10mmol...
Embodiment 2
[0069] 1) Preparation of diketone compounds.
[0070] Under the conditions of nitrogen, ice bath and mechanical stirring, take 21.62g (200mmol) of benzoquinone compound and 250mL of acetone in a 500mL three-necked flask, and dissolve 14.85mL (220mmol) of cyclopentadiene compound in 40mL of acetone in a constant pressure dropping funnel , under vigorous mechanical stirring, the acetone solution of cyclopentadiene was added dropwise to the acetone solution of 1,4-benzoquinone, kept for 1.5 hours, slowly raised to room temperature (25°C), and reacted at room temperature for 6 hours to obtain Tan solution. The acetone was removed by a rotary evaporator, and the remaining tan solid was recrystallized with n-hexane, and 30.92 g of a yellow needle-like crystal product, namely norbornene and cyclohexenedione compound, was precipitated.
[0071] (2) Preparation of dinitro compounds containing benzonorbornene structure.
[0072]Under the conditions of nitrogen and magnetic stirring, t...
Embodiment 3
[0080] 1) Preparation of diketone compounds.
[0081] Under the conditions of nitrogen, ice bath and mechanical stirring, take 21.62g (200mmol) of benzoquinone compound and 350mL of acetonitrile in a 500mL three-necked flask, and dissolve 16.2mL (240mmol) of cyclopentadiene compound in 60mL of acetonitrile in a constant pressure dropping funnel In , under vigorous mechanical stirring, the acetonitrile solution of cyclopentadiene was added dropwise to the acetonitrile solution of 1,4-benzoquinone, kept for 1.5 hours, slowly raised to room temperature (25°C), and reacted at room temperature for 6 hours to obtain Brown-yellow solution. The acetonitrile was removed by a rotary evaporator, and the remaining tan solid was recrystallized with n-hexane, and 29.48 g of a yellow needle-like crystal product, namely norbornene and cyclohexenedione compound, was precipitated.
[0082] (2) Preparation of dinitro compounds containing benzonorbornene structure.
[0083] Under the conditions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com