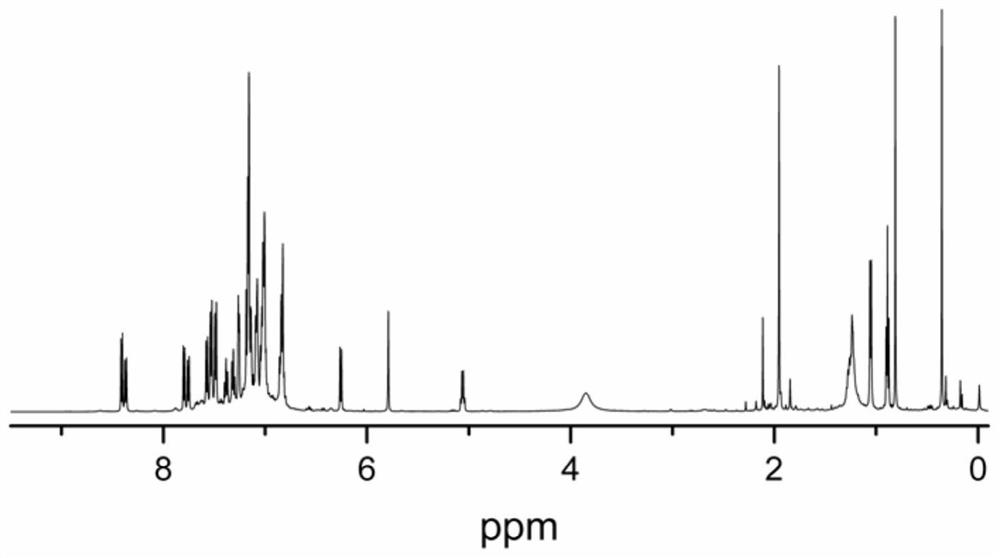
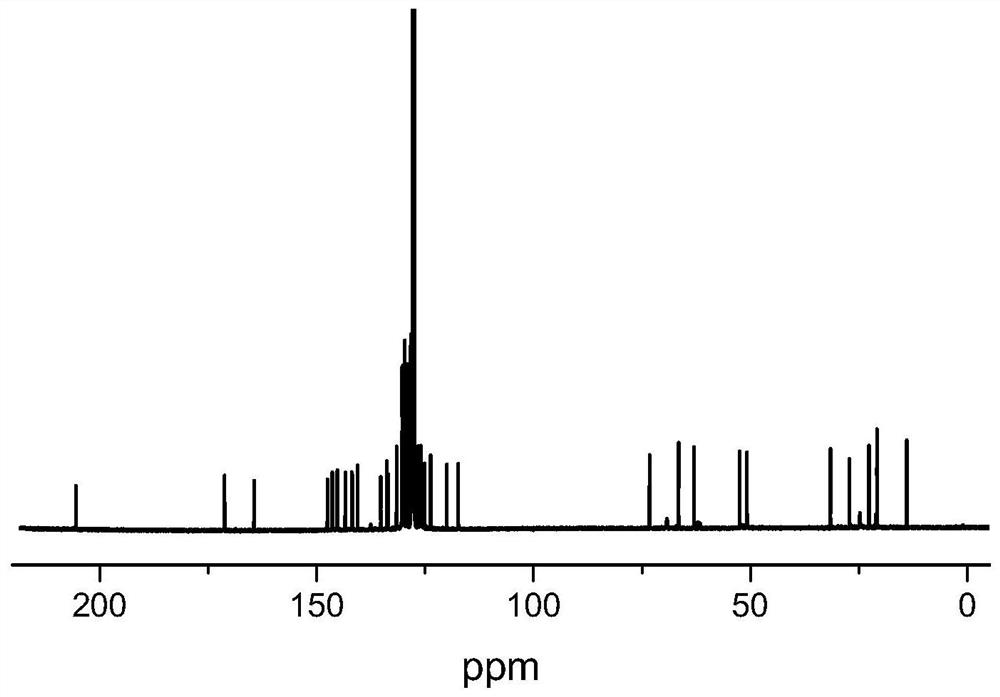
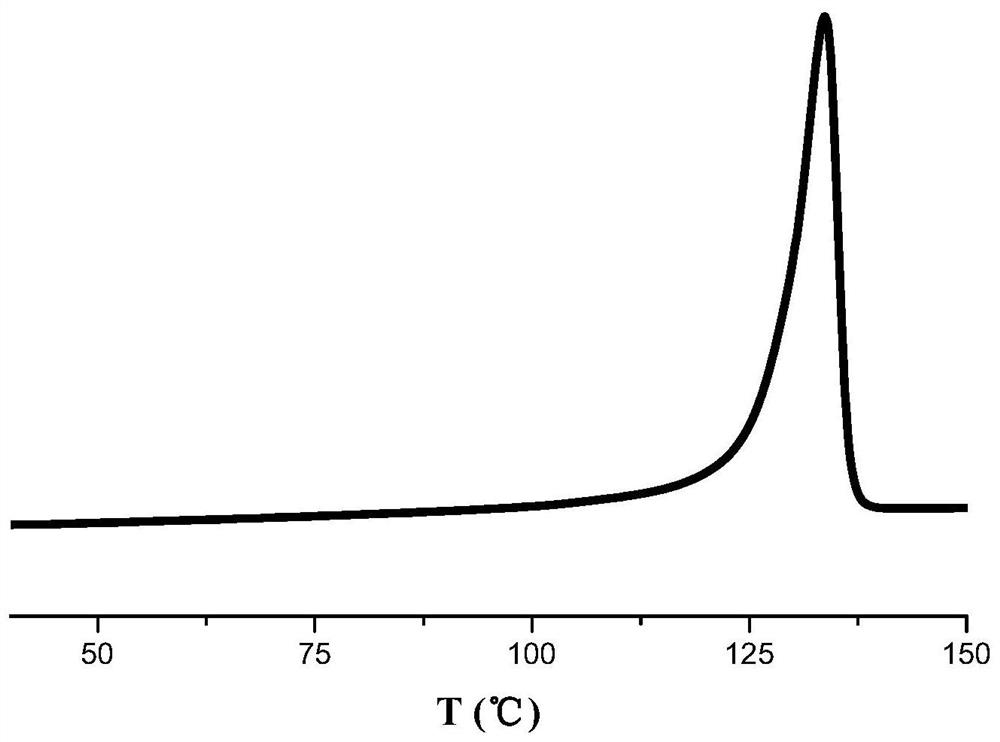
Pyridine amine hafnium compound as well as preparation method and application thereof
A technology of pyridylamine group and hafnium compound, applied in the field of pyridylamine group hafnium compound and preparation thereof, can solve the problem of no aryl group and the like, and achieve the effects of easy regulation, improved thermal stability and narrow molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Synthesis of pyridone compounds
[0070] Under a nitrogen atmosphere, 1.86g (10mmol) of 2-acetyl-6-bromopyridine, 1.72g (10mmol) of naphthaleneboronic acid, 15mg of tetrakis(triphenylphosphine)palladium, and 6g of potassium carbonate were successively added to the branch bottle, and injected 30mL ethanol, 20mL toluene, 10mL water, reflux overnight. Extract by liquid separation, wash with water, and dry to obtain 2.17 g of pyridine aldehyde compound A, with a yield of 93%. The H NMR spectrum is as follows: 1 H NMR (300MHz,C 6 D. 6 ):δ8.19-8.15(m,1H,ArH),8.01(dd,2.0Hz,1H,ArH),7.71-7.66(m,2H,ArH),7.45(dd,1H,ArH),7.33-7.28 (m,3H,ArH),7.18-7.13(m,2H,ArH),2.59(s,3H,(CH 3 )CO).
Embodiment 2
[0072] Synthesis of Substituted Aniline N1
[0073] Add 13.3g (72.2mmol) of benzhydryl alcohol and 3.66g (34mmol) of 4-methylaniline into the bottle, heat to 80°C to melt, add 2.4g (17.6mmol) of zinc chloride dissolved in hydrochloric acid, and heat up to 150°C. After reacting for 2 hours, cool down, dissolve with dichloromethane, neutralize excess acid with sodium bicarbonate, separate liquid extraction, spin dry, and rinse with ethanol to obtain 13.3 g of white powder with a yield of 89%. The H NMR spectrum is as follows: 1 H NMR (400MHz, CDCl 3 ):δ7.34-7.27(d,8H,Ph),7.24-7.18(d,4H,Ph),7.12-7.06(d,8H,Ph),6.38(s,2H,Ar-H),5.45( s,2H,CHPh 2 ),3.27(s,2H,NH 2 ), 2.02(s,3H,Me).
Embodiment 3
[0075] Synthesis of Substituted Aniline N2
[0076] According to the synthesis method in Example 2, the 4-methylaniline in Example 2 was replaced with 4-methoxyaniline, and the yield was 67%. The H NMR spectrum is as follows: 1 H NMR (400MHz, CDCl 3 ):δ3.24(2H,bs,NH 2 ),3.55(3H,s,OCH 3 ),5.64(2H,s,CHPh 2 ), 6.40(2H,s,Ar-H),7.25-7.43(20H,m,Ar-H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting temperature | aaaaa | aaaaa |
| Melting temperature | aaaaa | aaaaa |
| Melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com