Preparation method of high-thermal-conductivity super-hydrophobic phase change microcapsule
A phase-change microcapsule and superhydrophobic technology, applied in microcapsule preparations, microsphere preparation, heat exchange materials, etc., can solve the problems that cannot be used in applications, the hydrophobic effect is not obvious enough, and the size difference is not obvious enough, etc. The effect of reducing surface energy, improving thermal conductivity, and improving compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
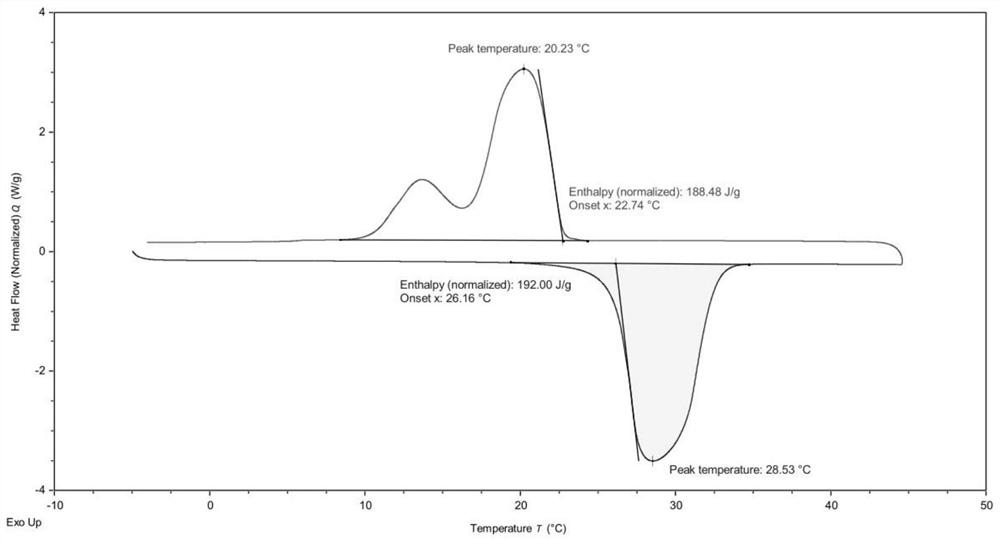
Image
Examples
Embodiment 1
[0030] 1) Preparation of modified inorganic nanoparticles: 5g of silane coupling agent γ-aminopropyltriethoxysilane (KH550) was dissolved in ethanol, configured into a solution with a mass fraction of 10%, and then added to 4g of nano-alumina In a mixed system with 20ml of ethanol, ultrasonically vibrate for 20min, continue stirring at room temperature for 3h, and finally filter the product and wash it several times with deionized water, then vacuum dry to obtain a white powder;
[0031] 2) Preparation of phase-change core material emulsion: Mix 100 g of styrene-maleic anhydride copolymer (SMA) with a solid content of 10% and 1 g of perfluorohexyl polyoxyethylene ether, add citric acid to adjust to PH=4.8, and heat up To 50°C, heat 80g of octadecane to make it melt into a liquid state, add it to the emulsifier, and disperse at high speed for 20 minutes to obtain a phase-change core material emulsion;
[0032] 3) Preparation of phase-change microcapsule emulsion: Add 2g of modi...
Embodiment 2
[0034]1) Preparation of modified inorganic nanoparticles: 4g of silane coupling agent 3-aminopropyltrimethoxysilane (KH551) was dissolved in ethanol, configured into a solution with a mass fraction of 5%, and then added to 4g of nano-aluminum nitride In a mixed system with 20ml of ethanol, ultrasonically vibrate for 40min, continue stirring at room temperature for 4h, and finally filter the product and wash it several times with deionized water, then vacuum dry to obtain a white powder;
[0035] 2) Preparation of phase-change core material emulsion: Mix 100 g of styrene-maleic anhydride copolymer (SMA) with a solid content of 10% and 2 g of perfluorooctyl polyoxyethylene ether, add acetic acid to adjust PH=6, and heat up to At 70°C, heat 80g of eicosane to make it melt into a liquid state, add it to the emulsifier, and disperse at high speed for 10 minutes to obtain a phase-change core material emulsion;
[0036] 3) Preparation of phase-change microcapsule emulsion: Add 3 g of...
Embodiment 3
[0038] 1) Preparation of modified inorganic nanoparticles: 4g of silane coupling agent 3-aminopropyltrimethoxysilane (KH551) was dissolved in ethanol, configured into a solution with a mass fraction of 2%, and then added to 4g of nano-silicon nitride In a mixed system with 20ml of ethanol, ultrasonically vibrate for 60min, continue stirring at room temperature for 2h, and finally filter the product and wash it several times with deionized water, then vacuum dry to obtain a white powder;
[0039] 2) Preparation of phase change core material emulsion: mix 100g of styrene-maleic anhydride copolymer (SMA) with a solid content of 10% and 3g of sodium perfluorononenyloxybenzenesulfonate evenly, add citric acid to adjust to PH= 4.8, heat up to 60°C, heat 80g of octadecane to make it melt into a liquid state, add it to the emulsifier, and disperse at high speed for 30 minutes to obtain a phase-change core material emulsion;
[0040] 3) Preparation of phase-change microcapsule emulsion...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| phase transition enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com