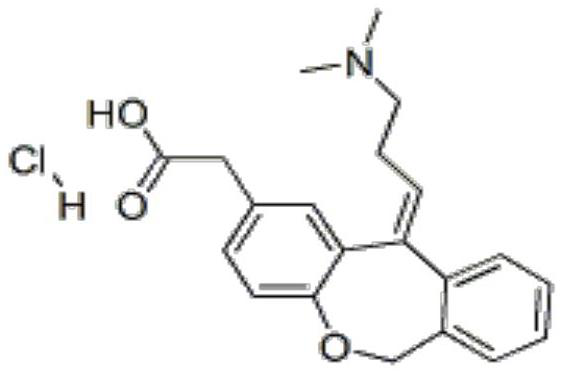
Preparation method of olopatadine hydrochloride
A technology of olopatadine hydrochloride and hydrobromide, which is applied in the field of drug synthesis and can solve the problems of long reaction routes and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
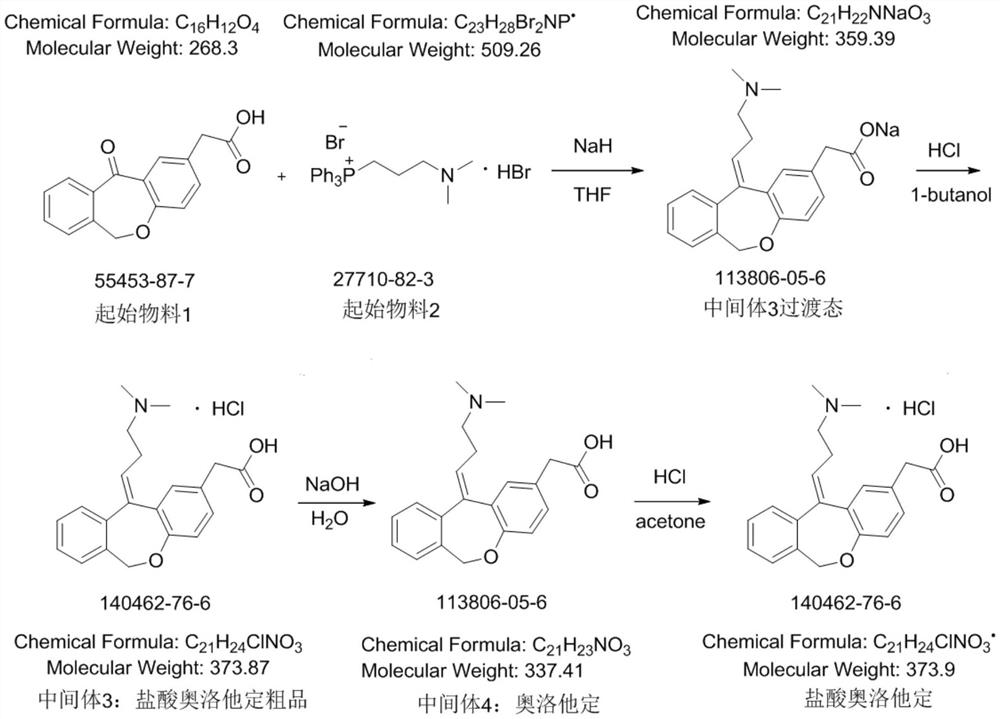
[0023] A, under nitrogen protection conditions, at first in 60.0kg massfraction is 10% THF, add 30.76kg (60.40mol) [3-(dimethylamino) propyl group] triphenylphosphine bromide hydrobromide, Control the temperature at 10°C, then add 3.65kg (152.12mol) of sodium hydride solution with a mass fraction of 60% and 3.96kg of dimethyl sulfoxide with a mass fraction of 0.66%, keep stirring at 20°C for 1 hour, then raise the temperature to 45 ℃, continue to stir for 2h, finally add 6.00kg (22.37mol) 11-oxo-6,11-dihydrodibenzo[b,e]oxazepine-2-acetic acid, continue to stir for 15h, until the reaction system turns black The reaction of the brown turbid liquid is finished, and the reaction liquid is quenched by a mixed solution of 77.94kg purified water and 2.44kg mass fraction of 10% tetrahydrofuran, and the aqueous phase is treated with a mixed solvent of 5kg hydrochloric acid and 87.4kg n-butanol, and the obtained aqueous solution is passed through Concentrate under reduced pressure, and ...
Embodiment 2
[0028] A, under nitrogen protection condition, at first in 52.34kg massfraction is 10% tetrahydrofuran, add 18.31kg (44.74mol) [3-(dimethylamino) propyl group] triphenyl phosphorus bromide hydrobromide, Control the temperature at 15°C, then add 2.59kg (107.71mol) of sodium hydride solution with a mass fraction of 60% and 3.12kg of dimethyl sulfoxide with a mass fraction of 0.66%, keep stirring at 23°C for 2 hours, and then heat up to 48°C , continue to stir for 2h, and finally add 6.00kg (22.37mol) of 11-oxo-6,11-dihydrodibenzo[b,e]oxazepine-2-acetic acid, continue to stir for 18h, until the reaction system turns dark brown The reaction of the turbid liquid is completed, and the reaction solution is quenched with a mixed solution of 62.41kg purified water and 1.83kg mass fraction of 10% tetrahydrofuran, and the aqueous phase is treated with a mixed solvent of 3.1kg hydrochloric acid and 64.23kg n-butanol, and the obtained aqueous solution is passed through Concentrate under re...
Embodiment 3
[0033] A, under nitrogen protection condition, at first in 68.25kg massfraction is 10% tetrahydrofuran, add 34.17kg (67.11mol) [3-(dimethylamino) propyl group] triphenyl phosphorus bromide hydrobromide, Control the temperature at 20°C, then add 4.118kg (173.99mol) of sodium hydride solution with a mass fraction of 60% and 5.12kg of dimethyl sulfoxide with a mass fraction of 0.66%, keep stirring at 30°C for 2 hours, and then heat up to 48°C , continue to stir for 2h, and finally add 6.00kg (22.37mol) of 11-oxo-6,11-dihydrodibenzo[b,e]oxazepine-2-acetic acid, continue to stir for 20h, until the reaction system turns dark brown The reaction of the turbid liquid is finished, and the reaction solution is quenched by a mixed solution of 93.12kg purified water and 3.67kg mass fraction of 10% tetrahydrofuran, and the aqueous phase is treated with a mixed solvent of 8kg hydrochloric acid and 98.12kg n-butanol, and the aqueous solution obtained is then subjected to reducing Concentrate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com