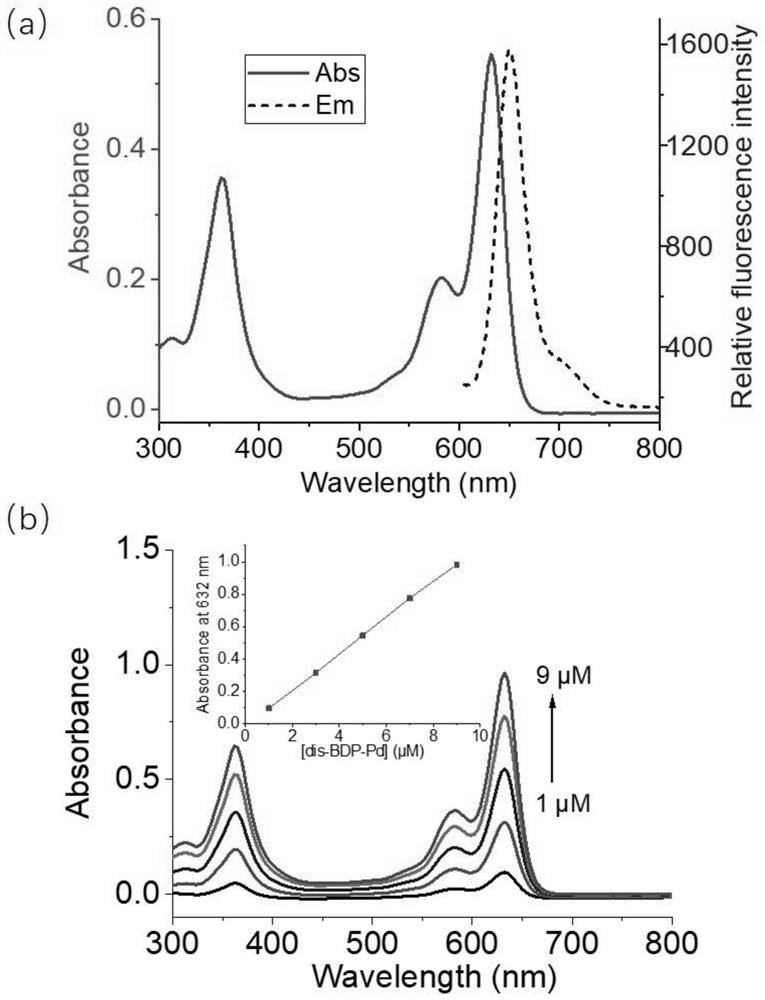
Near-infrared palladium ion fluorescent probe compound and synthesis method thereof
A near-infrared palladium ion fluorescent probe compound and its synthesis method technology, applied in the field of palladium ion detection and fluorescent probes, to achieve high sensitivity, high selectivity, and strong anti-interference effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of Compound 3
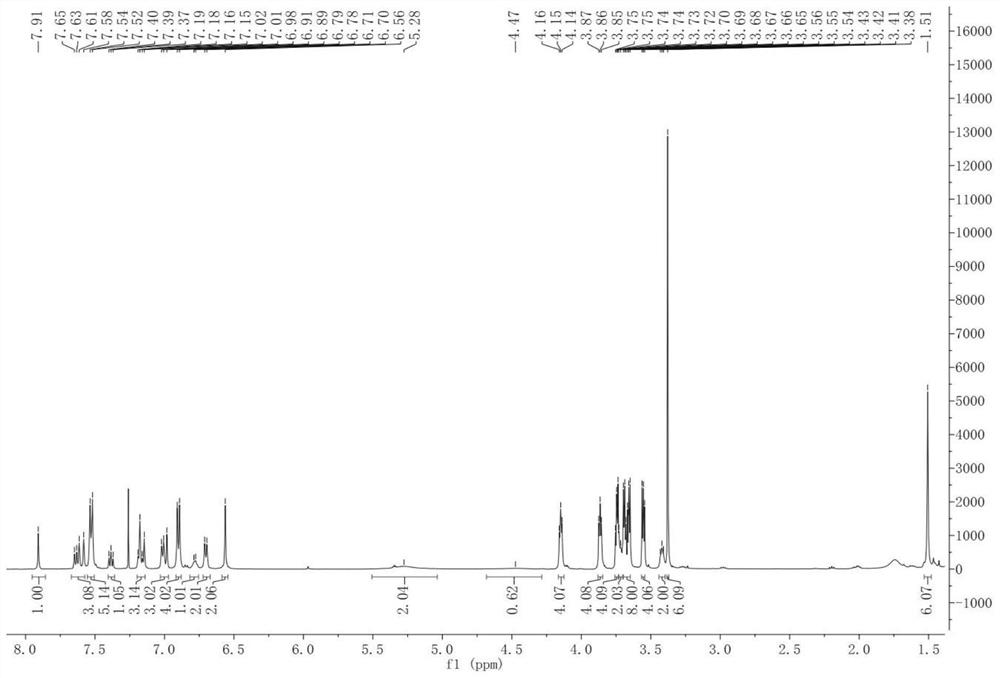
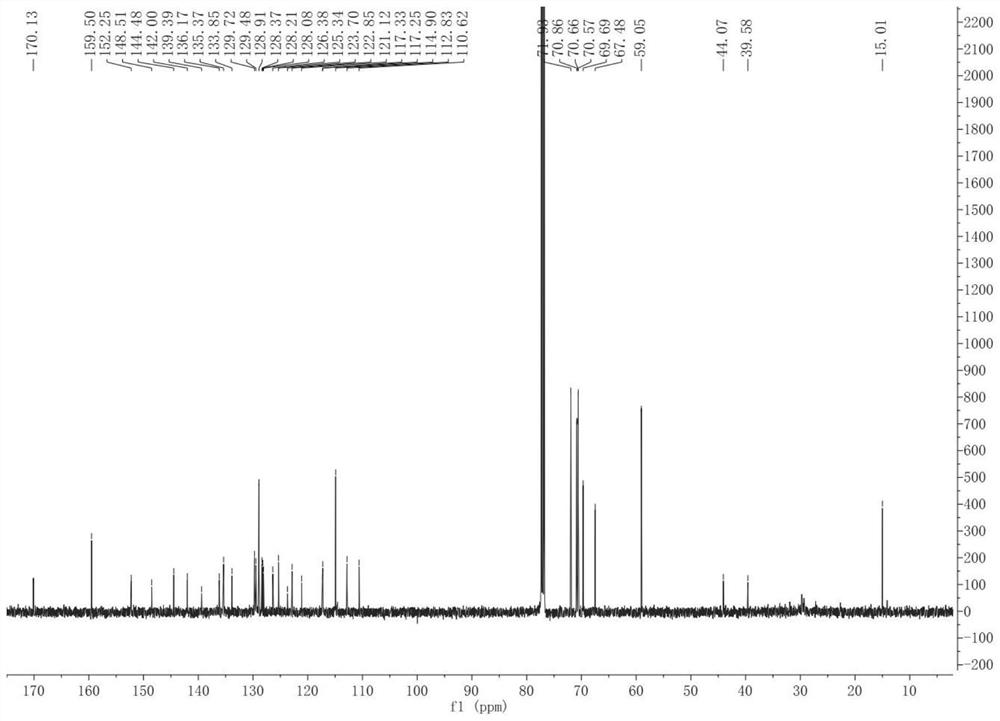
[0040] Under nitrogen protection, acetic acid was added to the compound 1 nitro BODIPY (800 mg, 2.16 mmol) and Compound 2 pairs of triethylene glycol monomethyl ether (2.32 g, 8.64 mmol) toluene (80 mL) (2.4 ml) ), Piperidine (8 mL) and catalytic amount of magnesium oxcetic acid, heating reflux for 5 h, Dean-Stark device is divided into water. After the reaction is completed, the solvent was removed under reduced pressure, and the crude product was purified by Ethyl acetate, and the silica gel column chromatography was purified. 1 H NMR (CDCL 3 : Δ8.36 (D, J = 8.3Hz, 2H, ARH), 7.51-7.62 (M, 8H, ARH AND CH), 7.21 (D, J = 16.3Hz, 2H, CH), 6.94 (D, J = 8.5Hz, 4H, ARH, 6.61 (S, 2H, Pyrrole-H), 4.17 (T, J = 4.8 Hz, 4H, CH 2 ), 3.87 (t, j = 4.8 Hz, 4h, CH 2 ), 3.72-3.77 (m, 4h, ch 2 ), 3.64-3.71 (m, 8h, ch 2 ), 3.54-3.56 (m, 4h, ch 2 ), 3.38 (s, 6h, ch 3 ), 1.39 (S, 6H, CH 3 . 13 C { 1 H} NMR (CDCL 3 : δ159.8, 153.4, 148.3, 142.4, 140.9, 136...
Embodiment 2
[0041] Example 2: Preparation of Compound 4
[0042] Under nitrogen protection, the zinc powder (960 mg, 15 mmol) was slowly added to the compound 3 (200 mg, 0.23 mmol), and the mixture was stirred at room temperature for 20 min. After the reaction is completed, the water is added (10 mL), ethyl acetate extraction (50 ml × 3), combined with organic phase, saturated NaHCO 3 Solution wash, no water NASO 4 Dry, filtration, evaporation solvent, and crude products were purified by ethyl acetate, silica gel column chromatography, and n-hexane / ethyl acetate re-crystalline dense blue solid compound 4 (150 mg, 78%). HNMR (CDCL 3 : Δ7.55-7.62 (M, 6H, ARH AND CH), 7.19 (D, J = 16.3 Hz, 2H, CH), 7.05 (D, J = 8.94 (D, J), 6.94 (D, J = 8.7Hz, 4H, ARH), 6.79 (D, J = 8.4 Hz, 2H, ARH), 6.66 (S, 2H, Pyrrole-H), 4.18 (T, J = 4.9 Hz, 4H, CH 2 ), 3.89 (t, j = 4.9 Hz, 4h, CH 2 ), 3.85 (S, 2H, NH 2 ), 3.74-3.78 (m, 4h, ch 2 ), 3.65-3.78 (m, 8h, ch 2 ), 3.54-3.59 (m, 4h, ch 2 ), 3.39 (s, 6h, ch 3 ), 1....
Embodiment 3
[0043] Example 3: Preparation of Compound 6
[0044] Under nitrogen protection, an appropriate amount of activated molecular sieve was added to 1,2-dichloroethane solution (10 mL) of Compound 4 (150 mg, 0.536 mmol) and Compound 5 (85.2 mg, 0.536 mmol). Add a vivo acetic acid, stirred at room temperature for 1 h, add NABH 3 CN (67.7 mg, 1.074 mmol) was continued at room temperature. After the reaction was completed, a saturated sodium hydrogencarbonate solution (10 ml), dichloromethane (3 × 25 mL) was added to the system. The combined organic phase was washed with water, saturated salt water, dried over anhydrous sodium sulfate, filtered, and the crude product obtained after evaporation solvent was dichloromethane / methanol (V : V = 45: 1) was purified by purifying liquid silica gel column chromatography, recrystallization to give a blue-black solid compound 6 (72.1 mg, 41%). 1 H NMR (CDCL 3 : Δ7.54-7.63 (M, 6H, ARH AND CH), 7.18 (D, J = 16.2 Hz, 2H, CH), 7.05 (D, J = 8.5 Hz, 2H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com