Method for preparing high-carbon alcohol from butene oligomer
A technology for oligomers and higher alcohols, applied in the field of preparing higher alcohols, can solve the problems of poor stability of heterogeneous catalysts, decreased reaction activity, catalyst deactivation, etc., achieves excellent reaction activity and selectivity, reduces separation costs, The effect of high economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Preparation of heterogeneous catalyst for hydroformylation
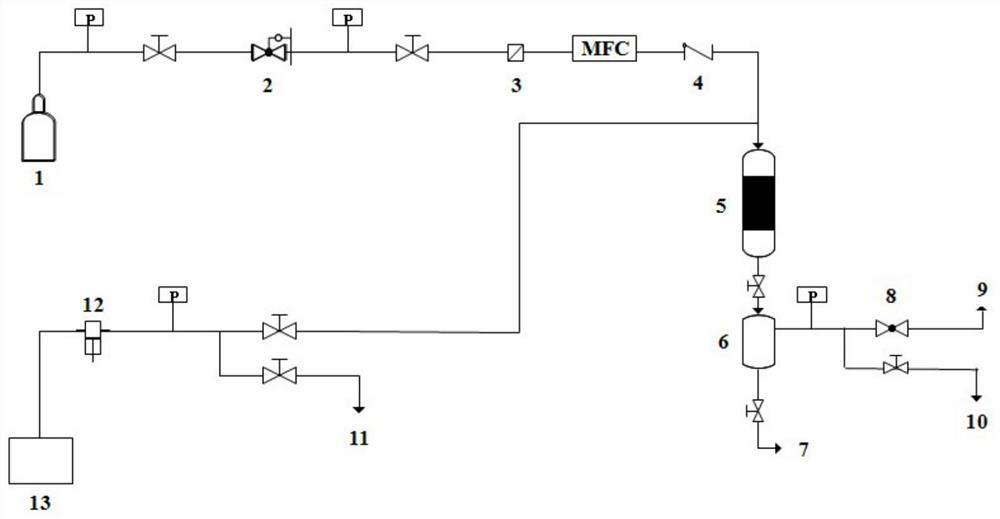
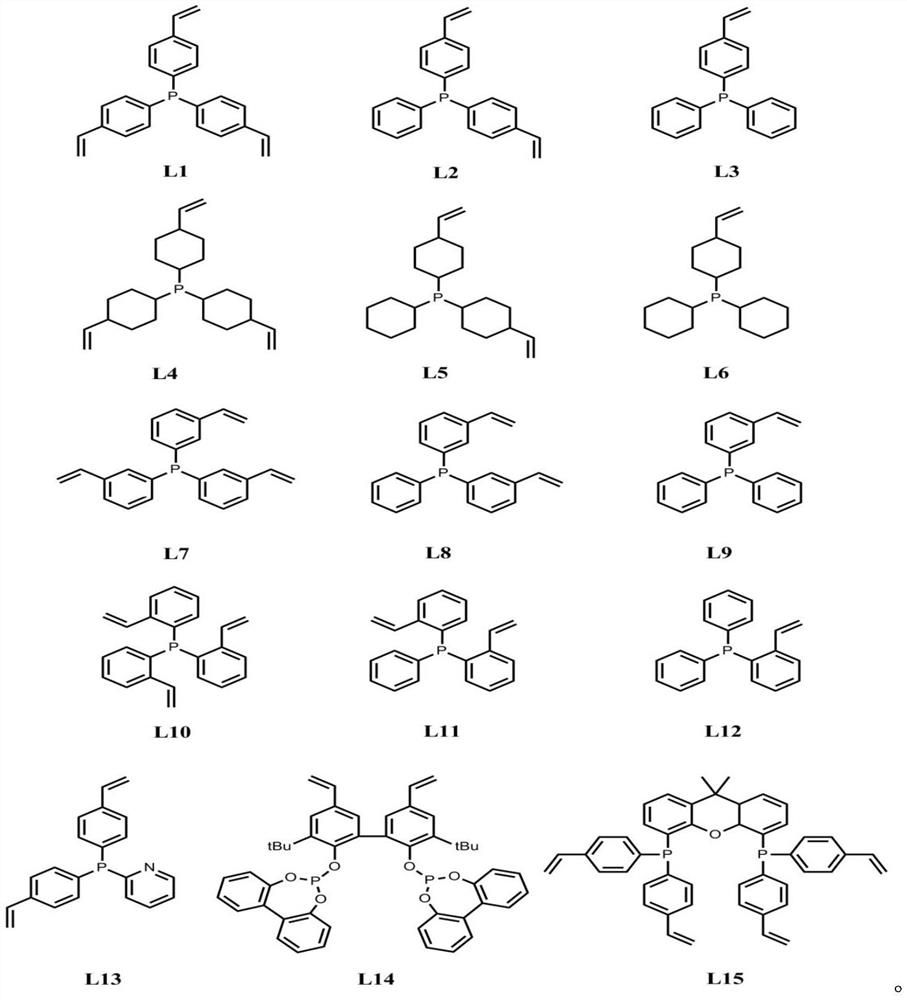
[0028] Under 298K and Ar gas protection atmosphere, 10.0g tris (4-vinylphenyl) phosphine ligand is dissolved in 100ml tetrahydrofuran solvent, adds 0.25g free radical initiator azobisisobutyronitrile in the above-mentioned solution, stirs 1h. The stirred solution was transferred to a hydrothermal autoclave, and polymerized by solvothermal method at 373K and Ar gas protection atmosphere for 24h. After the above-mentioned polymerization, cool to room temperature, and vacuum the solvent at 338K to obtain the organic phosphine ligand polymer (its specific surface area is 1080m 2 / g, the pore volume is 1.56m 3 / g, the pore size distribution is 0.12-90.0nm). Under an atmosphere of 298K and Ar gas protection, 62.65 mg of rhodium acetylacetonate dicarbonyl was weighed and dissolved in 100 ml of tetrahydrofuran solvent, 5.0 g of the phosphine ligand polymer prepared above was added, and stirred for 24 hours. Sub...
Embodiment 2
[0036] 1) Preparation of heterogeneous catalyst for hydroformylation
[0037] The preparation of the hydroformylation heterogeneous catalyst is the same as in Example 1.
[0038] 2) Butene oligomer hydroformylation reaction process
[0039] The hydroformylation heterogeneous catalyst prepared above is loaded into a fixed-bed reactor, and quartz sand is loaded at both ends. into the synthesis gas (H 2 :CO=1:1) and raw material butene oligomer (butene dimer content 98.8%, of which 2,4,4-trimethyl-1-pentene content 89.6%, 2,4,4-trimethyl-1-pentene content 89.6%, 2,4,4-trimethyl-1 The methyl-2-pentene content is 9.2%), the butene oligomer is transported into the reaction system by a high-pressure pump, and the synthesis gas is directly fed in the form of gas. At 373K, 3MPa, butene oligomer liquid hourly space velocity 2.0h -1 , Syngas gas space velocity 1000h -1 conditions for the hydroformylation reaction. The reaction product was collected at 2.5°C via a collection tank eq...
Embodiment 3
[0045] 1) Preparation of heterogeneous catalyst for hydroformylation
[0046] The preparation of the hydroformylation heterogeneous catalyst is the same as in Example 1.
[0047] 2) Butene oligomer hydroformylation reaction process
[0048] The hydroformylation heterogeneous catalyst prepared above is loaded into a fixed-bed reactor, and quartz sand is loaded at both ends. into the synthesis gas (H 2 :CO=1:1) and raw material butene oligomer (butene trimer content 95.2%), the butene oligomer is transported into the reaction system by a high-pressure pump, and the synthesis gas is directly fed in the form of gas. At 403K, 7MPa, butene oligomer liquid hourly space velocity 1.85h -1 , Syngas gas space velocity 1000h -1 conditions for the hydroformylation reaction. The reaction product was collected at 2.5°C via a collection tank equipped with circulating cooling. The obtained liquid phase product was analyzed by HP-7890N gas chromatography, and n-propanol was used as the in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore volume | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com