Iron-polysaccharide complex enteric capsule and preparation method thereof
A technology of enteric-coated capsules and complexes, which is applied in the field of medicine, can solve the problems of unfilled sustained and controlled release preparations, inconvenient administration methods, and unfavorable iron digestion and absorption, and achieve increased drug safety, low drug production costs, and gastric The effect of intestinal irritation is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
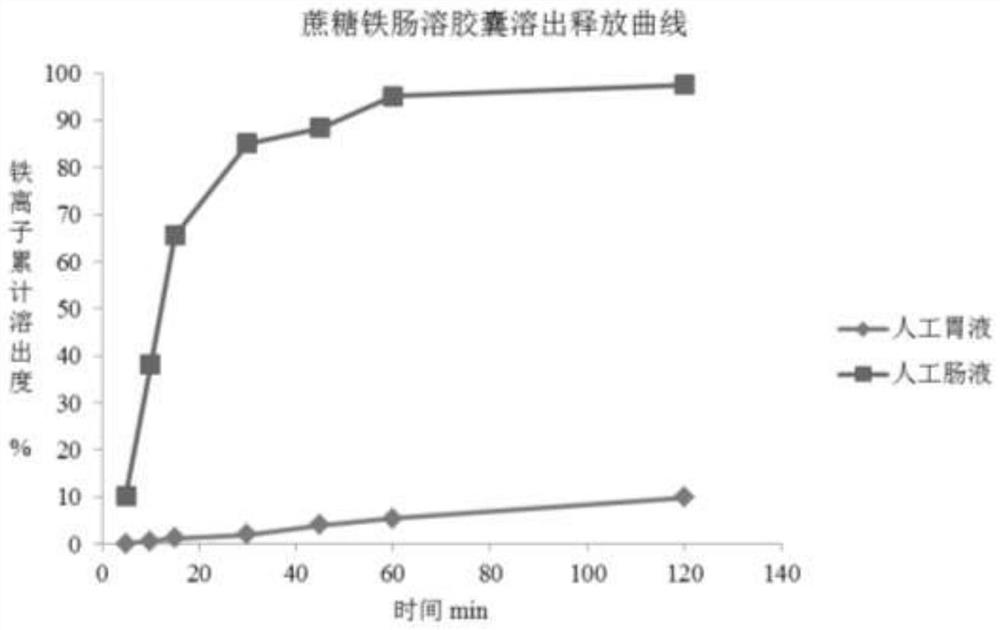
[0032] Example 1 Iron sucrose complex enteric-coated capsules
[0033] prescription
[0034]
[0035]
[0036] Preparation Process
[0037] ① Weigh the prescribed amount of carboxymethyl cellulose, prepare a solution with purified water, and pass through a 200-mesh sieve for later use.
[0038] ② Weigh the prescribed amount of sucrose core and place it in the centrifugal granulation coating machine, add the prescribed amount of iron sucrose and the pre-configured carboxymethyl cellulose solution to make pellets, pass through a 40-mesh sieve, and place in a high-efficiency boiling Boiling drying at 60°C in a dryer.
[0039] ③Weigh the polyacrylic acid resin, polyethylene glycol, and castor oil of the prescribed amount, prepare an ethanol solution, and pass through a 200-mesh sieve for later use.
[0040] ④Put the above-mentioned drug-containing pill cores into a centrifugal granulation coating machine, and use the above-mentioned coating solution to coat until the weig...
Embodiment 2
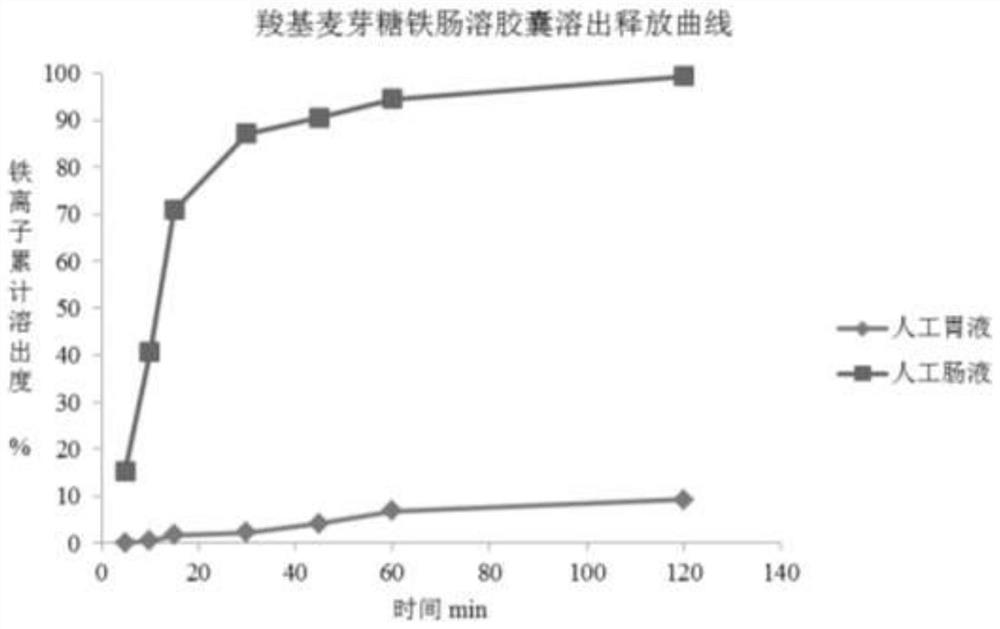
[0042] Example 2 Carboxymaltose iron complex enteric-coated capsules
[0043] prescription
[0044] name Dosage (mg) Proportion (%) Iron carboxy maltose 535.7 71.8 Cane sugar core 110.8 14.8 carboxymethyl cellulose 9.8 1.3 polyacrylic resin 76.2 10.2 polyethylene glycol 8.6 1.2 castor oil 4.9 0.7 Gelatin Empty Capsules 1 capsule /
[0045] Preparation Process
[0046] ① Weigh the prescribed amount of carboxymethyl cellulose, prepare a solution with purified water, and pass through a 200-mesh sieve for later use.
[0047] ② Weigh the prescribed amount of sucrose ball core and place it in the centrifugal granulation coating machine. At the same time, add the prescribed amount of carboxymaltose iron and the pre-configured carboxymethyl cellulose solution to make pellets. After passing through a 40-mesh sieve, place it in the flow Boiling and drying at 50°C in a chemical bed.
[0048] ③Weigh the polyacrylic aci...
Embodiment 3
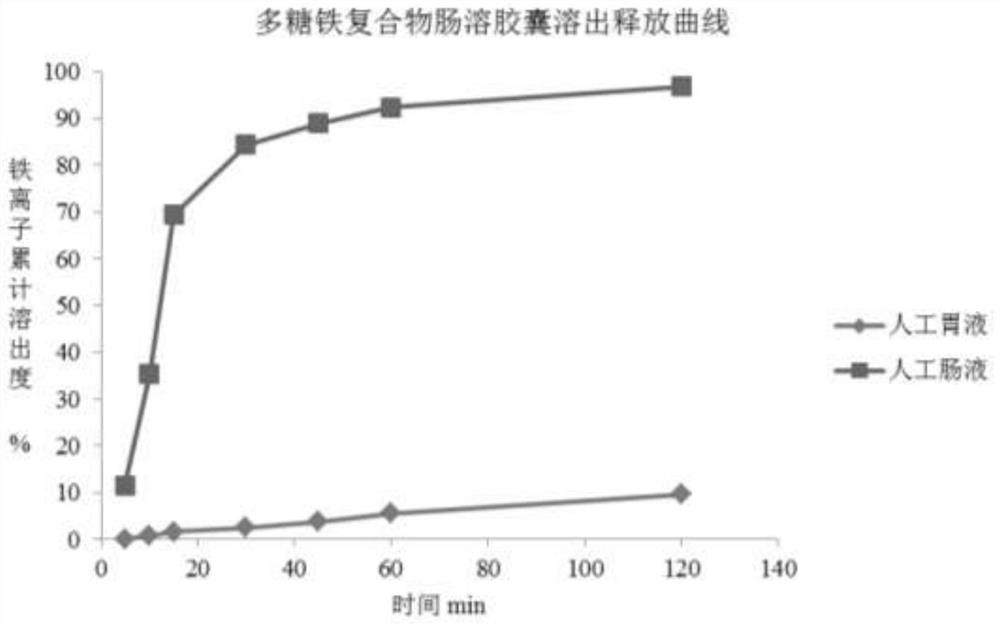
[0051] Example 3 Polysaccharide-iron complex enteric-coated capsules
[0052] prescription
[0053] name Dosage (mg) Proportion (%) polysaccharide iron complex 350.2 66.1 Cane sugar core 108.3 20.4 Povidone 5.5 1.0 polyacrylic resin 56.8 10.7 polyethylene glycol 6.8 1.3 triacetin 2.6 0.5 Gelatin Empty Capsules 1 capsule /
[0054] Preparation Process
[0055] ① Weigh the prescribed amount of povidone, prepare a solution with purified water, and pass through a 200-mesh sieve for later use.
[0056] ② Weigh the prescribed amount of sucrose core and place it in the centrifugal granulation coating machine. At the same time, add the prescribed amount of polysaccharide-iron complex and pre-configured povidone solution to make pellets. After passing through a 40-mesh sieve, place in a fluidized Boiling and drying at 50°C in the bed.
[0057] ③Weigh the polyacrylic acid resin, polyethylene glycol, and triacetin in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com