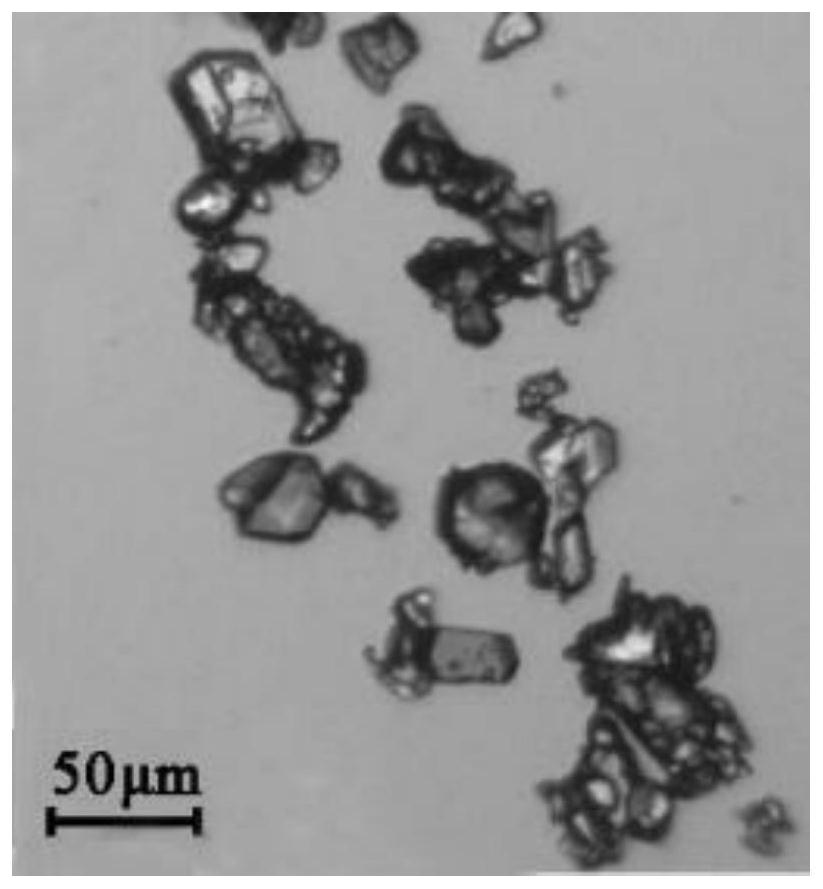
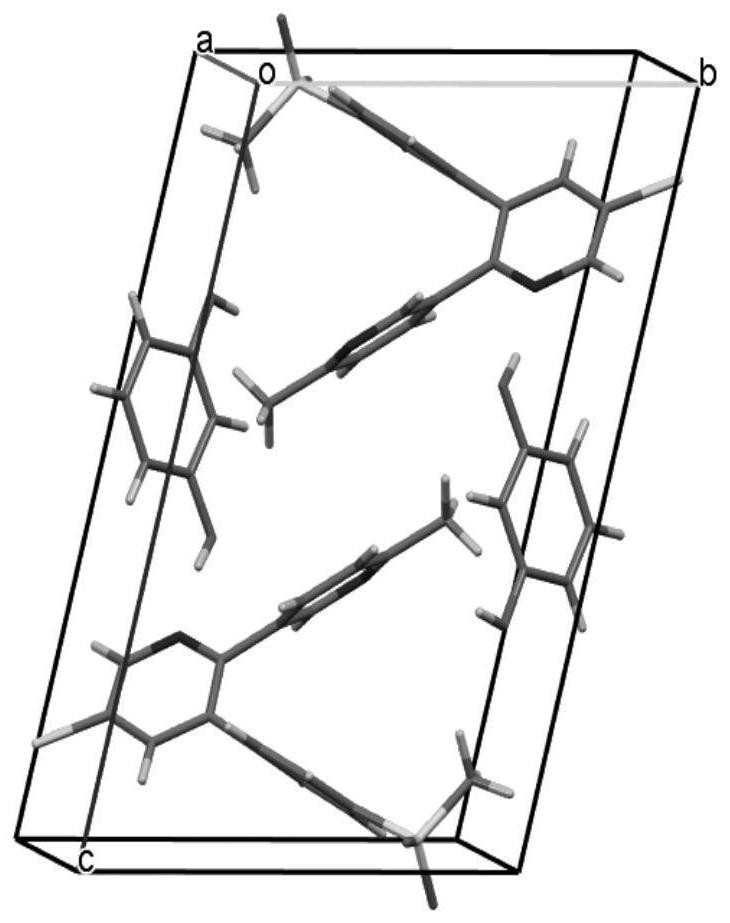
Etoricoxib-resorcinol pharmaceutical co-crystal and preparation method thereof
A technology of etoricoxib and resorcinol, which is applied in the field of etoricoxib-resorcinol drug co-crystal and its preparation, can solve the problems of low maximum dissolution concentration, complicated method, high energy consumption, etc., and achieve the improvement of biological Utilization, good product reproducibility, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Adopt cooling crystallization method to prepare etoricoxib-resorcinol drug co-crystal, the specific steps are as follows:
[0058] (1) Add 0.71g of etoricoxib crystal form I and 0.22g of resorcinol to 15.5mL of n-propanol, and heat to 60°C to form a clear solution;
[0059] (2) Cool the solution to 40°C at a rate of 30°C / h and maintain it for 10 minutes;
[0060] (3) Cool down to 0°C at a rate of 10°C / h and maintain for 2 hours;
[0061] (4) Then filter and dry the filter cake at 80°C.
[0062] The mol ratio of etoricoxib and resorcinol in the obtained etoricoxib-resorcinol drug cocrystal is 1:1, and cocrystal X-ray powder diffraction spectrum figure is as attached Figure 4 As shown, the diffraction angle 2θ is 5.68°, 9.42°, 15.56°, 15.88°, 17.14°, 17.74°, 18.26°, 20.06°, 20.40°, 20.94°, 21.32°, 21.58°, 22.56°, 23.16°, 23.48 °, 23.76°, 24.68°, 25.76°, 26.22°, 27.84°, 28.34°, 29.48°, 30.06° have characteristic peaks; its DSC spectrum is as attached Figure 5 As show...
Embodiment 2
[0064] Adopt cooling crystallization method to prepare etoricoxib-resorcinol drug co-crystal, the specific steps are as follows:
[0065] (1) Add 0.71g of the mixture of etoricoxib crystal form I and crystal form III, and 0.22g resorcinol to 10mL ethanol, and heat to 50°C to form a clear solution;
[0066] (2) Cool the solution to 30°C at a rate of 25°C / h and maintain it for 15 minutes;
[0067] (3) Cool down to 5°C at a rate of 8°C / h and maintain for 1.5h;
[0068] (4) Then filter and dry the filter cake at 65°C.
[0069] The mol ratio of etoricoxib and resorcinol in the gained etoricoxib-resorcinol drug cocrystal is 1:1, and the X-ray powder diffraction spectrum figure of cocrystal is as attached Figure 4 As shown, the diffraction angle 2θ is 5.58°, 9.32°, 15.36°, 15.78°, 17.04°, 17.54°, 18.19°, 20.00°, 20.30°, 20.90°, 21.30°, 21.68°, 22.32°, 23.11°, 23.40 °, 23.70 °, 24.88 °, 25.55 °, 26.22 °, 27.74 °, 28.25 °, 29.37 °, 30.01 ° have characteristic peaks; its DSC spectru...
Embodiment 3
[0071] Adopt cooling crystallization method to prepare etoricoxib-resorcinol drug co-crystal, the specific steps are as follows:
[0072] (1) Add 1.80 g of etoricoxib crystalline form I and crystalline form V mixture, 0.58 g resorcinol to 6.0 mL dimethyl carbonate, and heat to 40°C to form a clear solution;
[0073] (2) Cool the solution to 35°C at a rate of 20°C / h and maintain it for 20 minutes;
[0074] (3) Cool down to 10°C at a rate of 5°C / h and maintain for 1h;
[0075] (4) Then filter and dry the filter cake at 50°C.
[0076] The mol ratio of etoricoxib and resorcinol in the gained etoricoxib-resorcinol drug cocrystal is 1:1, and the X-ray powder diffraction spectrum figure of cocrystal is as attached Figure 4 As shown, the diffraction angle 2θ is 5.88°, 9.62°, 15.61°, 15.98°, 1.94°, 17.94°, 18.35°, 20.24°, 20.40°, 20.74°, 21.32°, 21.78°, 22.52°, 23.16°, 23.48 °, 23.76 °, 24.78 °, 25.63 °, 26.29 °, 27.64 °, 28.54 °, 29.53 °, and 30.16 ° have characteristic peaks; its...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com