Waste tire pyrolytic carbon catalyst, preparation method and application
A technology for waste tires and waste tire particles, which is applied in the field of high-efficiency electrolytic water hydrogen evolution reaction catalyst and its preparation, and electrolytic water hydrogen evolution reaction. Simple, responsive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation method of the high-efficiency hydrogen evolution electrocatalyst for the pyrolysis of waste tires in this example is as follows:
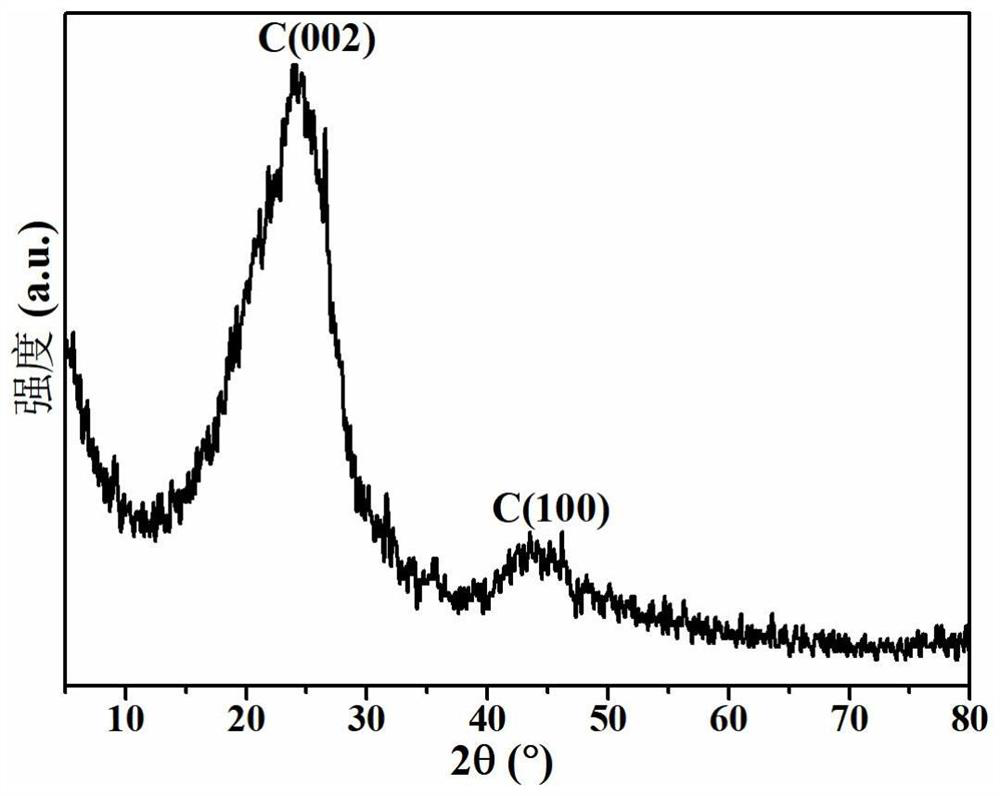
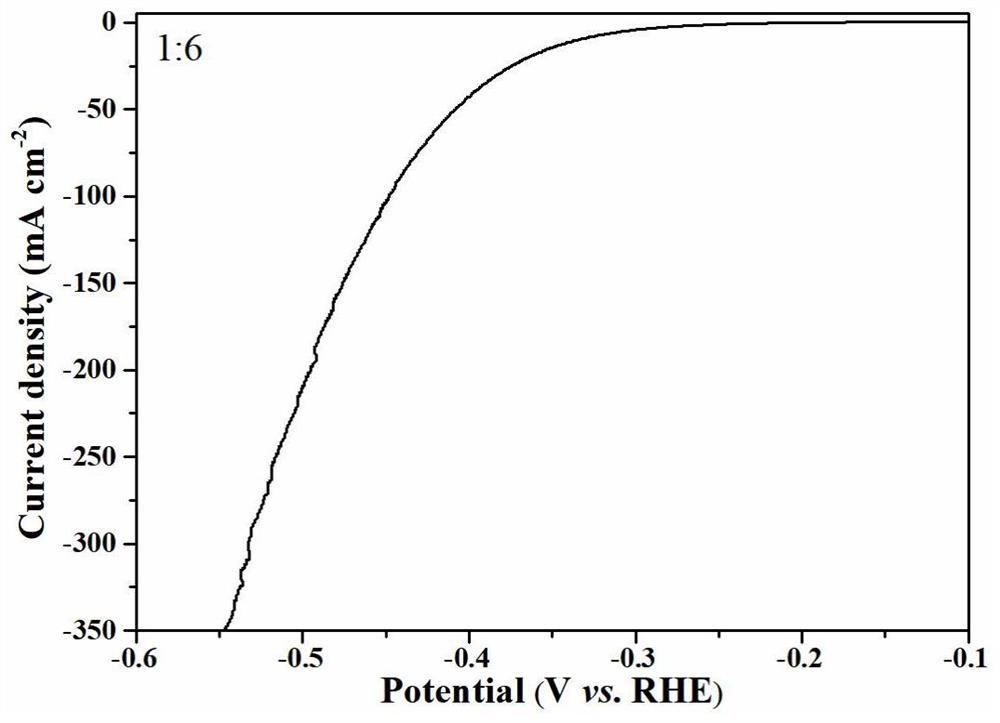
[0030] Fully mix the crushed waste tire particles and ammonium chloride at a mass ratio of 1:6, place them in a tube furnace, and heat them to 400°C at a heating rate of 1°C / min under a nitrogen atmosphere, and maintain them for 2 hours. Continue heating to 900°C at a heating rate of 2°C / min and maintain it for 2h, then use 2M HCl-20wt% HF to treat at 30°C for 12h, wash and dry to obtain the required pyrolysis carbon catalyst, mainly including mass The fraction is about 94.84% C, 2.22% S and 2.94% N, and the specific surface area is about 85m 2 / g.
[0031] The glassy carbon electrode was polished to a smooth surface with 0.05 μm alumina polishing powder, and then dried naturally for use. At the same time, 5 mg of the prepared catalyst was weighed, and after adding 5 wt % Nafion solution (30 μL) and ethanol (970 μL), the mi...
Embodiment 2
[0033] The preparation method of the high-efficiency hydrogen evolution electrocatalyst for the pyrolysis of waste tires in this example is as follows:
[0034]Fully mix the crushed waste tire particles and ammonium chloride at a mass ratio of 1:4, place them in a tube furnace, and heat them to 400°C at a heating rate of 0.5°C / min under a nitrogen atmosphere for 2 hours. Continue heating to 900°C at a heating rate of 2°C / min and maintain for 2h, then use 1M HCl-10wt% HF to treat at 60°C for 12h, wash, dry and cool to room temperature to obtain the required pyrolysis carbon catalyst. It mainly includes about 95.15% C by mass fraction, 2.23% S and 2.65% N, and the specific surface area is about 60m 2 / g.
[0035] The electrochemical performance measurement results show that the waste tire pyrolysis carbon electrocatalyst shows good catalytic performance for the hydrogen evolution reaction of electrolyzed water, and the current density is up to 134mA in the range of reduction po...
Embodiment 3
[0037] The preparation method of the high-efficiency hydrogen evolution electrocatalyst for the pyrolysis of waste tires in this example is as follows:
[0038] Fully mix the crushed waste tire particles and ammonium chloride at a mass ratio of 1:10, place them in a tube furnace, and heat them to 450°C at a heating rate of 1°C / min under a nitrogen atmosphere for 2 hours. Continue heating to 900°C at a heating rate of 2°C / min, and maintain it for 2h, then use 2MHCl-20wt%HF to treat at 60°C for 8h, wash, dry and cool to room temperature to obtain the required pyrolysis carbon catalyst. It mainly includes about 95% C by mass fraction, 2.15% S and 2.85% N, and the specific surface area is about 79m 2 / g.
[0039] The electrochemical performance measurement results show that the waste tire pyrolysis carbon electrocatalyst shows good catalytic performance for the hydrogen evolution reaction of electrolyzed water, and the current density is up to 290mA in the range of reduction pote...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com