Method for preparing foamy carbon supported FeNi alloy catalyst from nitro-modified metal organic framework and application of foamy carbon supported FeNi alloy catalyst
A metal-organic framework and alloy catalyst technology, applied in the application field of electrocatalytic water splitting catalysts, can solve problems such as agglomeration, uneven particle distribution, and limited catalytic performance of materials, and achieve low cost, wide source of raw materials, and excellent catalytic performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1FeNi@NCF
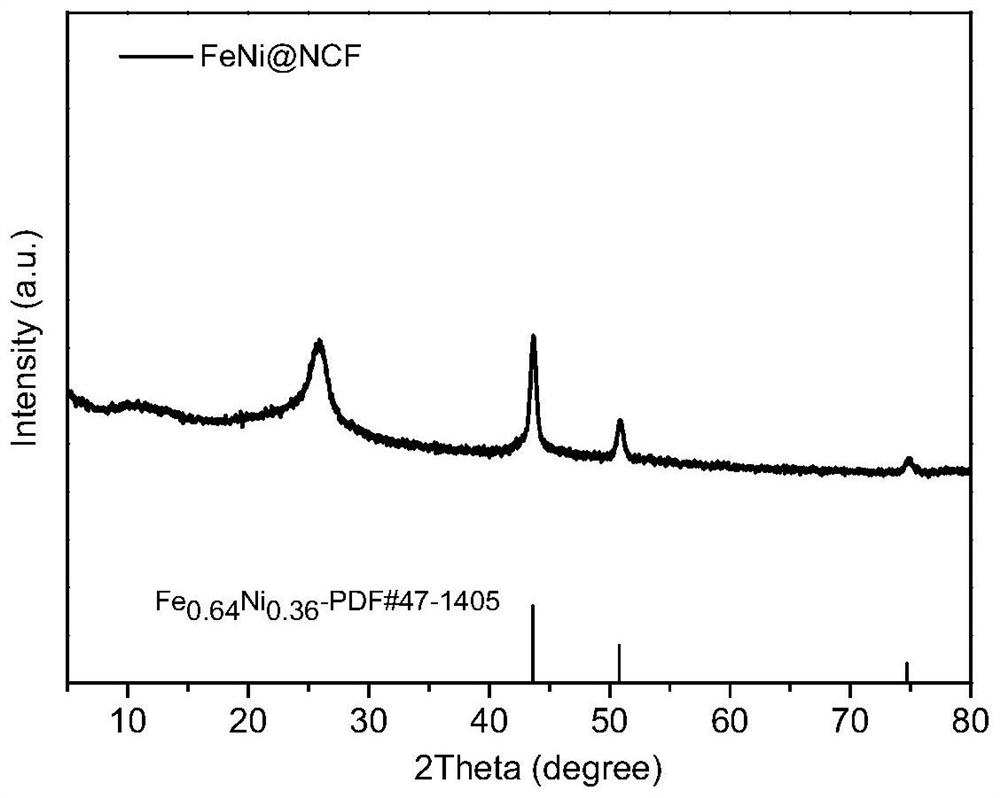
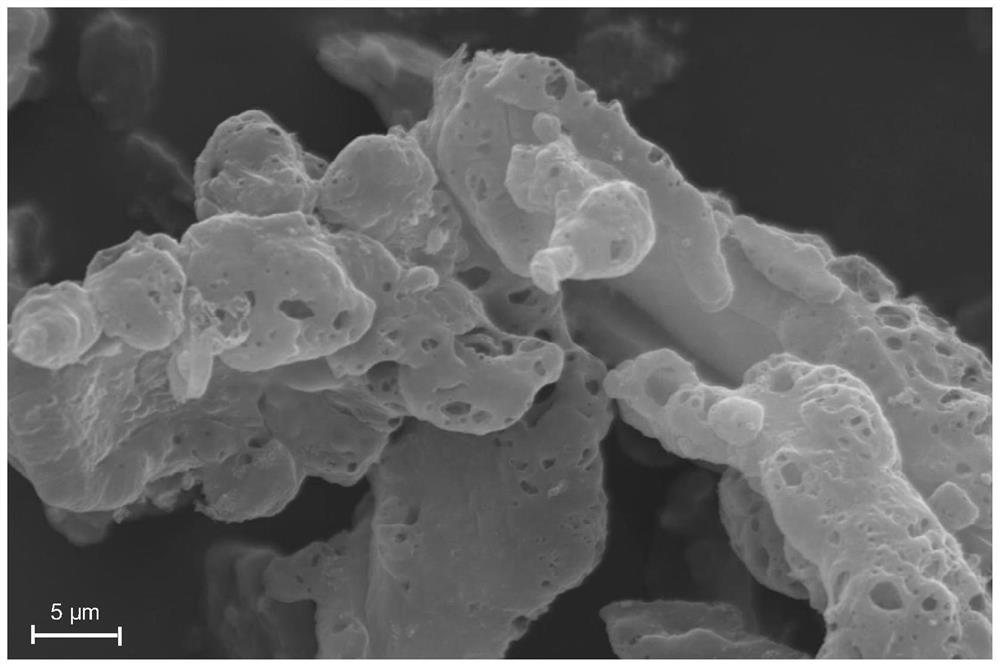
[0035] Weigh 0.5-1.0g of 5-nitroisophthalic acid and 4,4'-bipyridyl into 40-50mL of DMF solution, then add 0.05-1.2g of Zn(NO 3 ) 2 .6H 2 O, Ni(NO 3 ) 2 .6H 2 O and Fe(NO 3 ) 3 .9H 2 O. Then react under an inert atmosphere at a temperature of 100-130° C. for 20-30 hours, cool to room temperature, filter, and dry in a vacuum oven. Finally, the dried sample was raised to 800-1000°C at a heating rate of 5°C / min in a tube furnace, kept at a constant temperature for 30 minutes, and then cooled to room temperature to obtain a sample FeNi@NCF. The X-ray diffraction pattern of the product is shown in figure 1 ; Scanning Electron Microscopy, see figure 2 ; transmission electron microscope image see image 3 ; Nitrogen adsorption curve and pore size distribution diagram are shown in Figure 4 .
Embodiment 2
[0036] Example 2 Electrocatalytic OER performance test of FeNi@NCF
[0037] The electrocatalytic OER performance test of FeNi@NCF obtained in Example 1 was performed on a CHI760E electrochemical workstation at room temperature using a classic three-electrode system for electrochemical testing. The electrolyte is 1.0M KOH solution. Hg / HgO and Pt sheets were used as reference and counter electrodes. Take 2mg FeNi@NCF, add 150uL ultrapure water, 150uL isopropanol and 25uL Nafion177, and after ultrasonic for 1 hour, drop the sample on the glassy carbon electrode as the working electrode. Figure 5 The linear sweep voltammetry curve shown is obtained at a scan rate of 5mV / s. It can be seen from the figure that FeNi@NCF drives 10mAcm on the glassy carbon electrode -2 The required overpotential for the current density is 270mV, respectively. Image 6 The Tafel plot shown is from Figure 5 It is calculated that the Tafel slope of FeNi@NCF on the glassy carbon electrode is 67mV dec...
Embodiment 3
[0038] Example 3 FeNi@NCF electrochemical specific surface area test
[0039] To determine the electrochemical surface area (ECSA), cyclic voltammetry (CV) measurements were used to explore the electrochemical double layer capacitance (C dl ). CV is performed in the non-Faraday range (1.10-1.20Vvs RHE) with sweep rates of 20, 60, 100, 140 and 180mV s -1. A linearity plot was obtained by plotting the current density versus scan rate at 1.15 Vvs RHE. C dl is half the slope of the linear graph and is used to represent ECSA. The electrochemical specific surface area diagram is shown in Figure 8 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com