Catalyst for preparing ethylene by reducing carbon dioxide, catalytic electrode and preparation method
A carbon dioxide and catalytic electrode technology, which is applied in the direction of electrodes, electrolytic components, electrolytic processes, etc., can solve the problems that cannot meet the requirements of practical applications, and achieve the effect of high ethylene selectivity and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] In a second aspect, the present invention provides a method for preparing the catalyst for reducing carbon dioxide to ethylene, comprising the steps of:
[0035] Surface pretreatment of nano carbon to make it rich in surface functional groups;
[0036] Surface functionalized nano-carbon and nano-cuprous oxide are uniformly dispersed in a solvent in proportion and dried.
[0037] In some embodiments, the method for surface pretreatment of nano-carbon is: after mixing nano-carbon with oxygen source, nitrogen source, sulfur source, phosphorus source and / or halogen source, further treatment, that is, a surface rich in functional groups Nano carbon.
[0038] Further, the further treatment method is chemical treatment, heat treatment, oxygen / ammonia plasma treatment, high energy electron beam oxidation or ozone oxidation.
[0039] Further, the oxygen source is selected from oxygen, oxygen plasma, nitric acid, sulfuric acid, hydrogen peroxide, sodium hydroxide / potassium salt...
Embodiment 1
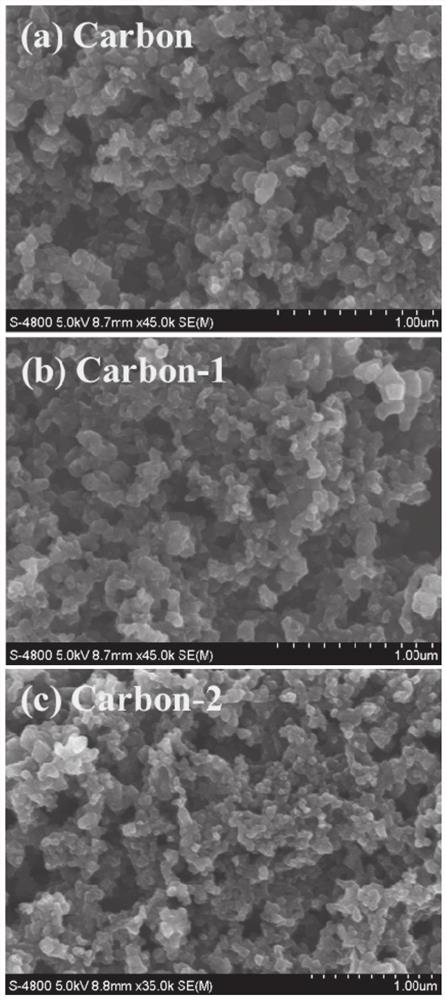
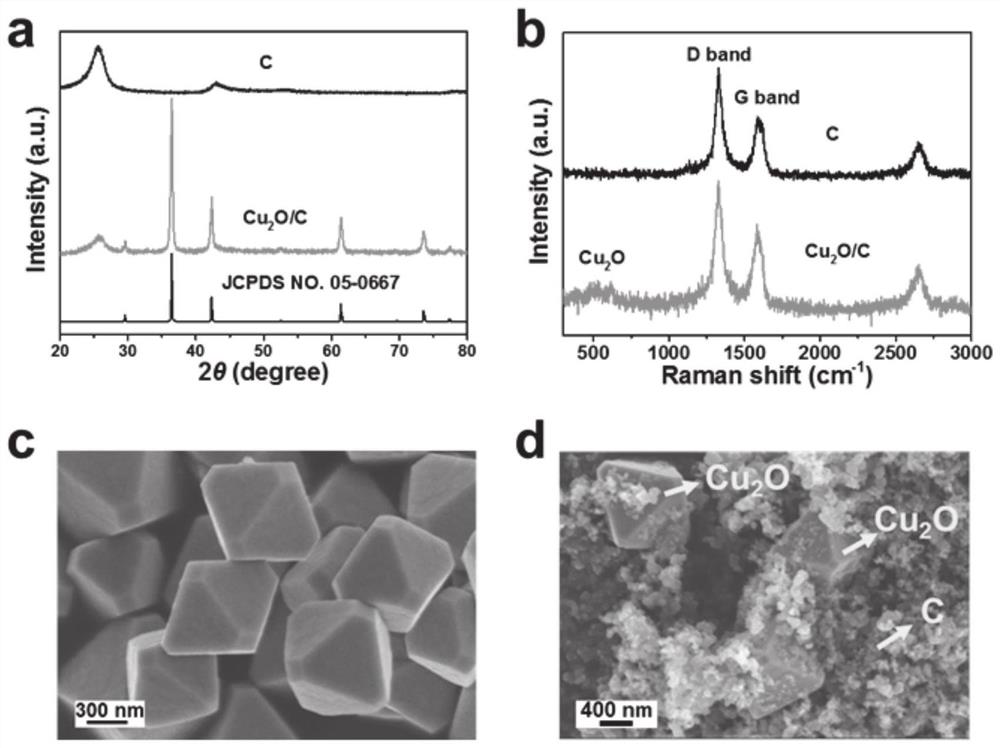
[0065] The carbon black nanoparticles were dispersed in 20ml of 0.5M nitric acid aqueous solution, and ultrasonicated at 10°C for 3h to obtain a uniformly dispersed mixed solution. Then the mixed solution was transferred to a 50ml polytetrafluoroethylene reaction kettle. Fix the polytetrafluoroethylene reaction kettle in a stainless steel autoclave, place it in an oven at 200°C, and heat it with water for 3 hours. After the reaction, the temperature of the reactor was naturally lowered, and the obtained mixed solution was subjected to centrifugal separation and deionized water washing treatment. Finally, the carbon material was placed in a vacuum drying oven at 60° C. and dried for 6 hours to obtain a carbon material after surface oxidation. Such as figure 1 As shown in b, it can be seen that the carbon material is in the shape of nanospheres.
[0066] The cuprous oxide nanoparticles of 200nm and the above pretreated carbon material were mixed in a solution consisting of is...
Embodiment 2
[0072] The difference from Example 1 is that the concentration of the aqueous nitric acid solution is 5M, and the others are the same as in Example 1.
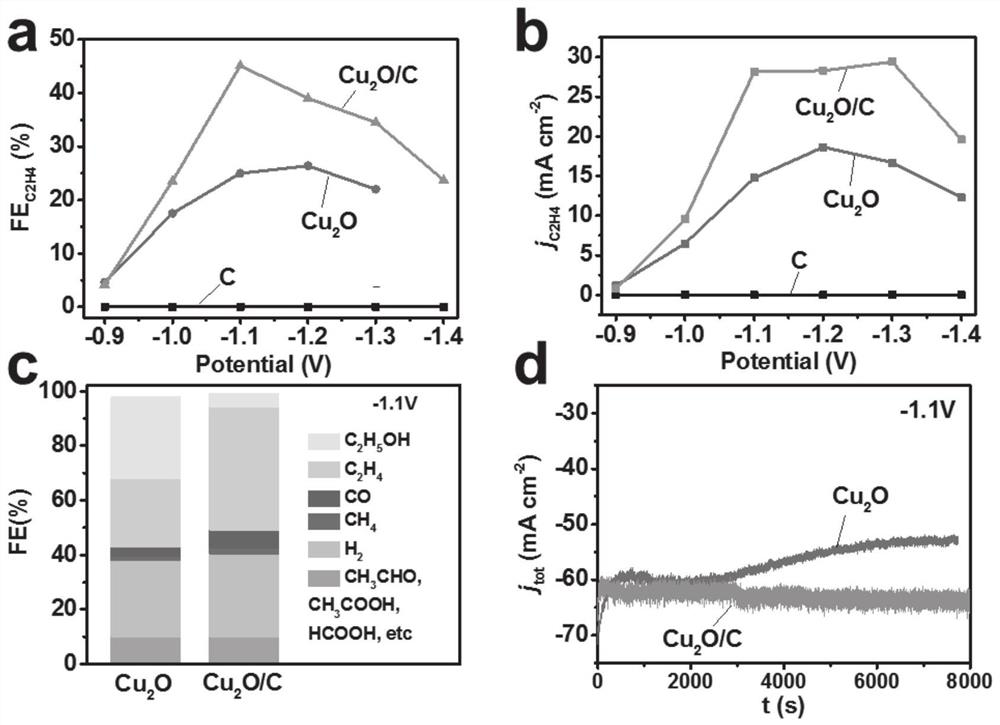
[0073] In a three-electrode system, at -1.1V vs RHE, the prepared C / Cu 2 O composite electrode for carbon dioxide reduction performance test, C / Cu 2 The ethylene Faradaic efficiency and current density of the O composite electrode are 70%, 50mA cm -2 . This is due to the increase of oxygen-containing groups on the carbon surface after the concentration of nitric acid increases. The oxygen groups on the surface of carbon can promote the bonding between its cuprous oxides, while inhibiting the production of ethanol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com