Method for preparing trimethylolpropane through hydrogenation with high condensation yield
A technology for preparing trimethylolpropane and hydrogen, applied in the field of hydrogenation synthesis, can solve the problems of increased product cost, many reaction by-products, difficult product separation, etc., so as to avoid device corrosion, reduce device material, and improve economy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
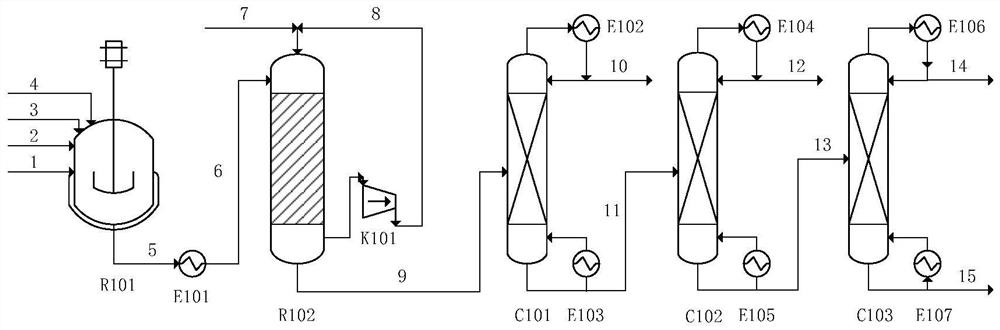
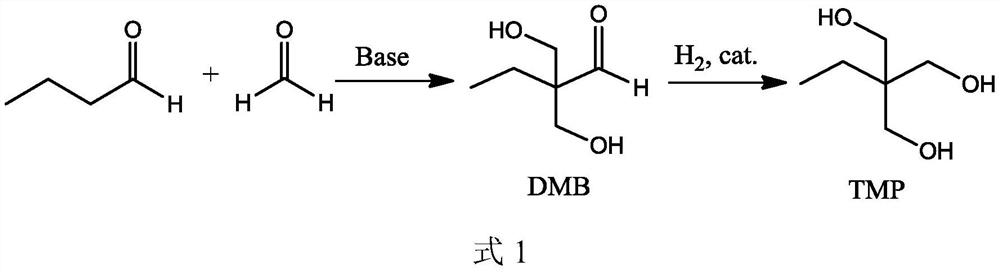
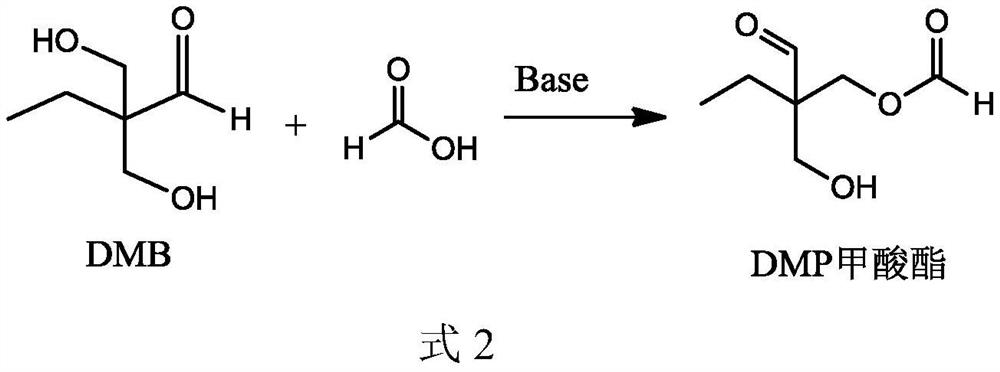
[0059] 37wt% formaldehyde, n-butyraldehyde, 5% catalyst aqueous solution, and water are reacted at 40°C in a molar ratio of formaldehyde, n-butyraldehyde, monosodium ethanolamine phosphate, and water at 40°C, and the reaction pressure The gauge pressure is 0.5MPa, the volume of the reactor is 10L (the effective volume of the liquid is 6L), and the residence time is 2h to obtain the condensation reaction solution 3 (stream 5) containing DMB, in which ethanolamine phosphate monosodium salt and N-ethyl di The molar ratio of ethanolamine is 1:5.
[0060] The condensation reaction solution 3 enters the fixed-bed hydrogenation reactor, and under the catalysis of Johnson Matthey Cu 2912 catalyst, DMB is hydrogenated to generate a hydrogenation reaction solution rich in trimethylolpropane (stream 9). The hydrogenation reaction temperature is 100°C, the reaction pressure is 5MPa gauge pressure, and the space velocity is 0.5mL / cm 2 cat. / h, the hydrogen-to-oil ratio (the molar ratio of ...
Embodiment 2
[0067] 37wt% formaldehyde, n-butyraldehyde, 5% catalyst aqueous solution, and water are reacted at 60°C in a molar ratio of formaldehyde, n-butyraldehyde, monopotassium ethanolamine phosphate, and water at 60°C, and the reaction pressure The gauge pressure is 0.3MPa, the volume of the reactor is 10L (the effective volume of the liquid is 6L), and the residence time is 1h to obtain the condensation reaction solution 4 (stream 5) containing DMB, wherein ethanolamine phosphate monopotassium salt and N-ethyl di The molar ratio of ethanolamine is 1:2.
[0068] The condensation reaction liquid 4 enters the fixed-bed hydrogenation reactor, and under the catalysis of Johnson Matthey Cu 4000T catalyst, DMB is hydrogenated to generate a hydrogenation reaction liquid rich in trimethylolpropane (stream 9). The hydrogenation reaction temperature is 160°C, the reaction pressure is 1MPa gauge pressure, and the space velocity is 5mL / cm 2 cat. / h, hydrogen oil ratio (molar ratio) is 4.
[006...
Embodiment 3
[0076] 50wt% formaldehyde, n-butyraldehyde, 4% catalyst aqueous solution and water are reacted at 50°C in a molar ratio of formaldehyde, n-butyraldehyde, ethanolamine phosphate monosodium salt and water at 2.5:1:0.01:1.781, and the reaction pressure The gauge pressure is 0.1MPa, the volume of the reactor is 10L (the liquid effective volume is 6L), the residence time is 3h, and the condensation reaction solution 5 (stream 5) containing DMB is obtained, wherein ethanolamine phosphate monosodium salt and N-ethyl di The molar ratio of ethanolamine is 1:10.
[0077] The condensation reaction liquid 5 enters the fixed-bed hydrogenation reactor, and under the catalysis of the Cu60 / 35T catalyst, DMB is hydrogenated to generate a hydrogenation reaction liquid rich in trimethylolpropane (stream 9). The hydrogenation reaction temperature is 130°C, the reaction pressure is 2MPa gauge pressure, and the space velocity is 3mL / cm 2 cat. / h, hydrogen oil ratio (molar ratio) is 3.
[0078]The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com