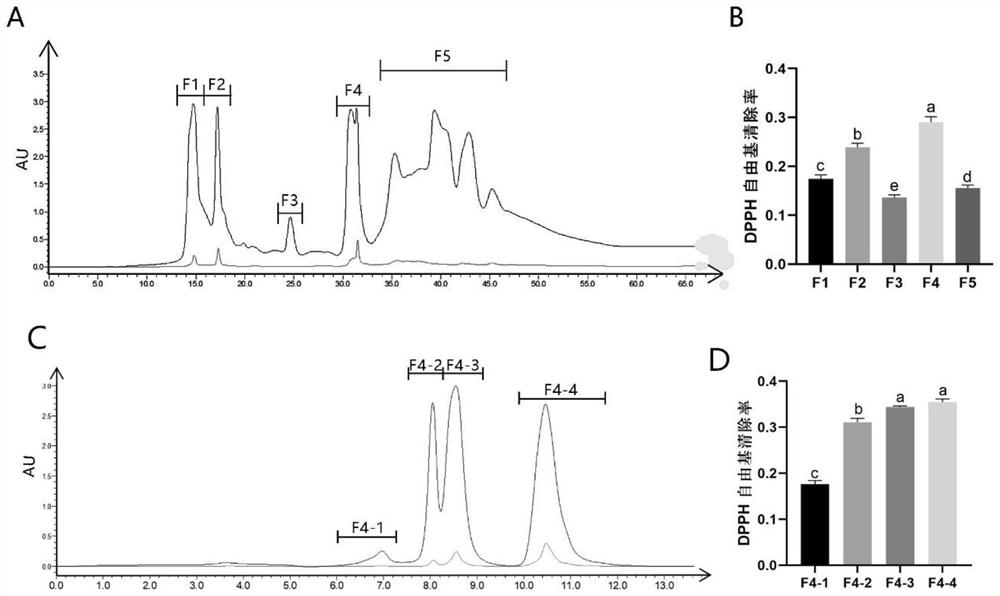
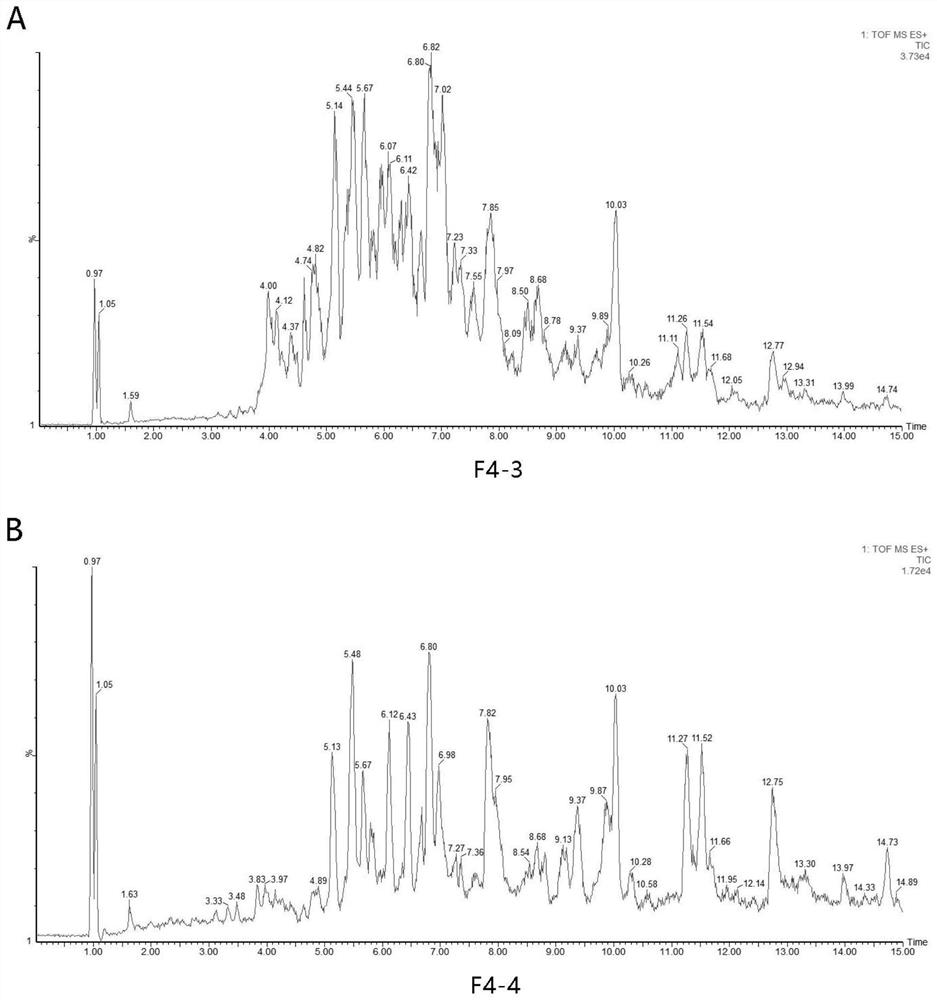
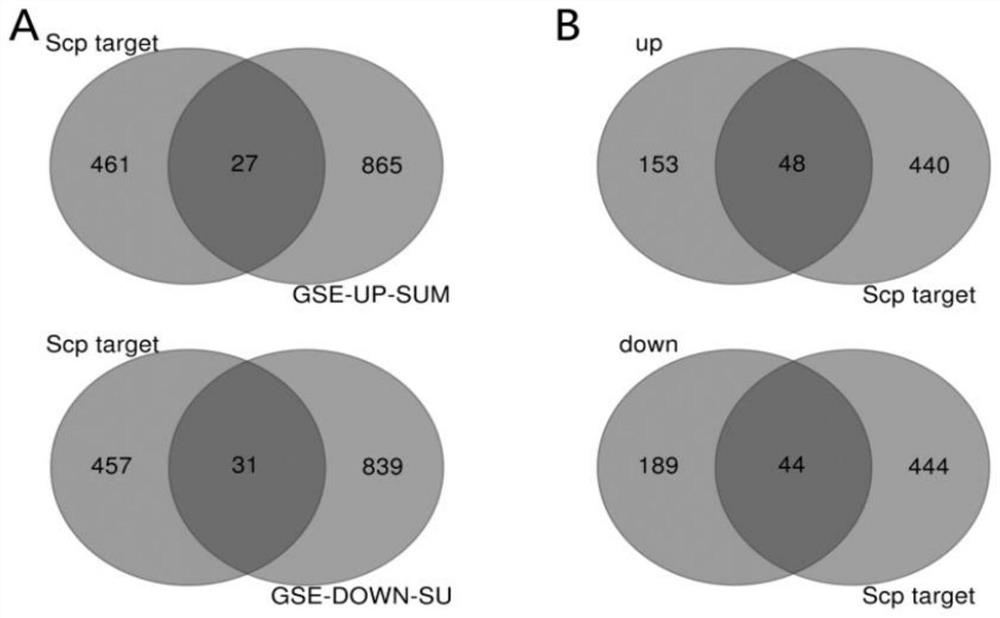
Screening method of functional peptide
A functional peptide and screening method technology, applied in the biological field, can solve the problems of large consumption of reagents, high cost, and long time consumption of functional peptides, and achieve the effects of reducing reagent consumption and pollution, increasing yield, and scientific methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A method for screening sea cucumber anti-fatigue peptides, comprising the following steps performed in sequence:
[0046] S1: call the regulatory network related to the anti-fatigue function; find out the first gene target set related to the anti-fatigue function according to the regulatory network. The specific operation is: obtain sports training and fatigue-related transcriptome data sets from the GEO database; use the enrichment analysis clusterProfiler data package to perform the first GO analysis on the transcriptome data sets, and find out the significant differences in expressions involved in endurance training The biological process of the study was used as the first set of gene targets related to fatigue resistance. When performing the first GO analysis, it specifically includes: according to the transcriptome data set, using p<0.05 as the screening condition, classify the differential genes whose expression is up-regulated or down-regulated into the first gen...
Embodiment 2
[0057] The preparation process of the peptide collection of anti-fatigue sea cucumber peptide is as follows:
[0058] Sea cucumber protein is hydrolyzed by protease, and hydrolyzed at 37-50° C. for 3-7 hours to obtain sea cucumber peptide hydrolyzate, wherein the mass ratio of protease to sea cucumber protein is 0.4-0.9:1. The protease is one or more of trypsin, neutral protease and alkaline protease.
[0059] Preferably, when the protease is composed of alkaline protease, trypsin and neutral protease, the mass ratio of the alkaline protease, trypsin and neutral protease is (0.5~1):(1~2): (1~2). The enzyme activity of the alkaline protease is 50,000-500,000 U / g, the enzyme activity of the trypsin is 100,000-1,000,000 U / g, and the enzyme activity of the neutral protease is 100,000-650,000 U / g.
[0060] Next, the sea cucumber peptide hydrolyzate is ultrafiltered with a 2000D ultrafiltration membrane to obtain small molecule peptides with a molecular weight below 2000D.
[006...
Embodiment 3
[0068] Example 3 Zoological verification of sea cucumber anti-fatigue peptide
[0069] The weight-bearing swimming test was carried out on the adult mice who were orally synthesized candidate peptides for 3 to 5 weeks. group (1.4mg / g·d) and high sea cucumber peptide dose group (2.8mg / g·d). Specifically: the weight is fixed by adding 5% body weight lead skin to the tail of the mouse. The mouse is placed in a 50×50cm water tank with a water depth of 50cm and a water temperature of 28°C±0.5°C. Swim for 60 minutes. After swimming, each mouse is wiped dry. The body was anesthetized with pentobarbital sodium (50mg / kg BW, i.p.), and blood, heart, muscle, and liver tissues were collected; 10 rats in each group.
[0070] The candidate peptides include LPGSDDF, FD(Hyp)GA, APGLTY and G(Hyp)LQADY.
[0071] Test items include blood sugar before and after exercise, serum lactic acid before and after exercise, blood urea nitrogen before and after exercise, MtDNA content in heart and skelet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com