Whole-cell synthesis method of D-psicose by taking glycerol as substrate
A technology of allulose and synthesis method, which is applied in the field of whole-cell synthesis of D-psicose, can solve problems such as difficult large-scale in vitro synthesis, expensive D-glyceraldehyde, troublesome separation and purification, etc., to avoid Purification steps and cofactors, easy separation and purification, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Transformation of glycerol into D-psicose by recombinant Escherichia coli whole cell method
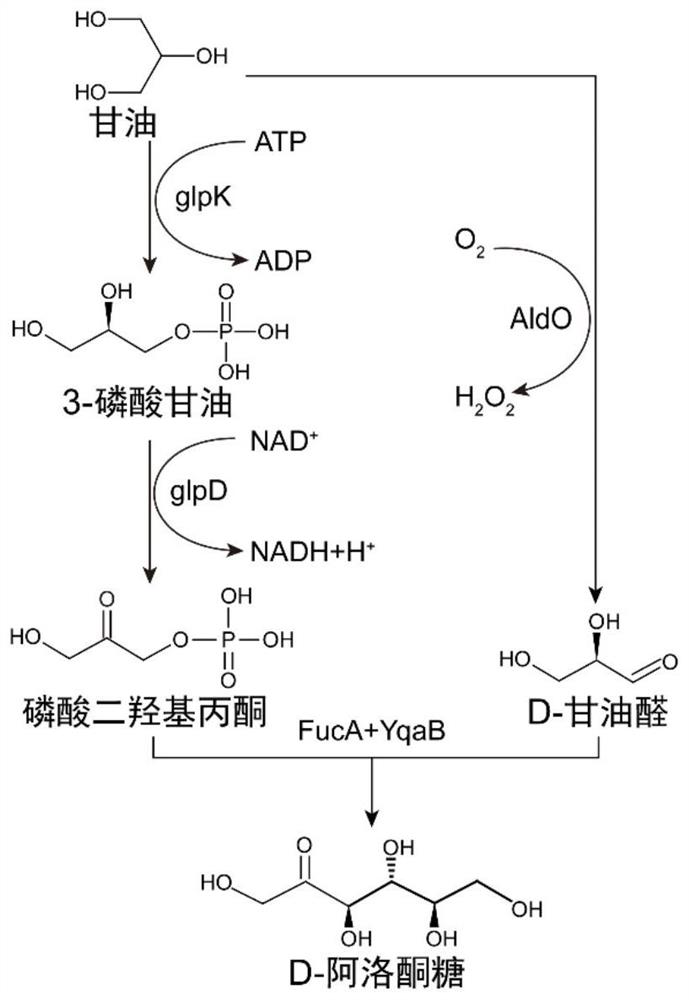
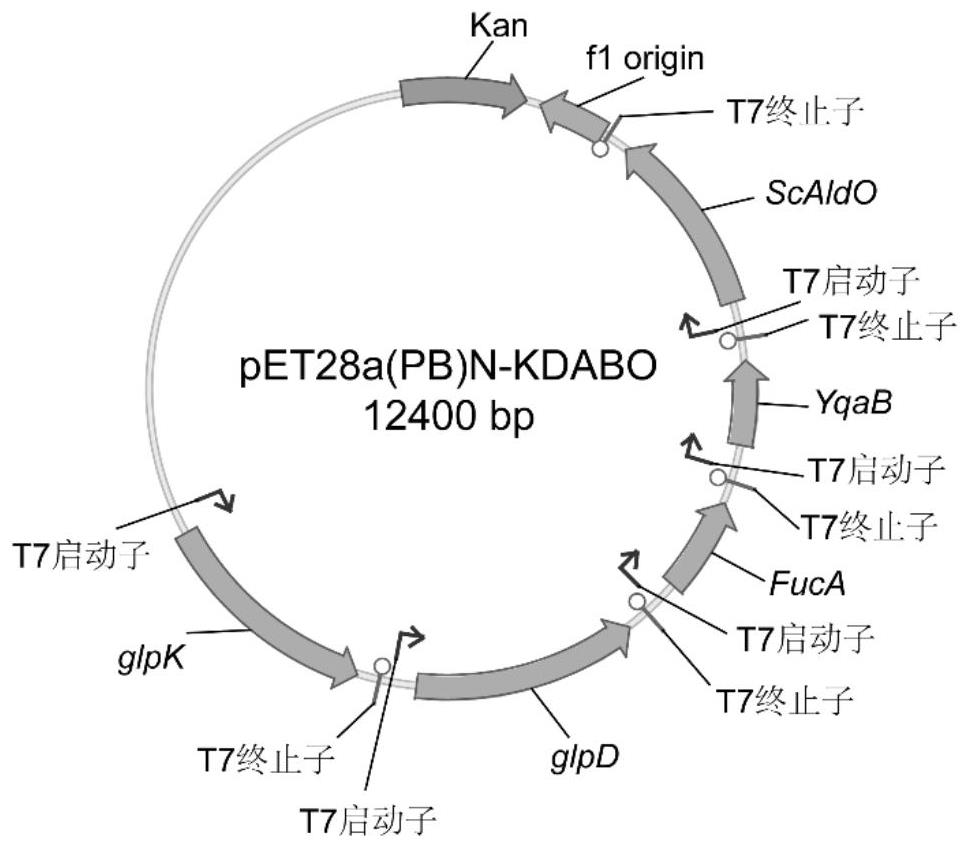
[0027] The glycerol kinase encoding gene (glpK, SEQ ID NO: 6), the glycerol 3-phosphate dehydrogenase encoding gene (glpD, SEQ ID NO: 7), the L-fucose-1-phosphate derived from Escherichia coli MG1655 Aldolase encoding gene (FucA, SEQ ID NO: 8) and fructose-1-phosphorylase encoding gene (YqaB, SEQ ID NO: 9) and sugar alcohol oxidase encoding gene (AldO, SEQ ID NO: 10), were cloned between the BamHI and HindIII sites of the vector pET28a (PB) using a one-step cloning kit (Nanjing Novizan Biotechnology Co., Ltd.) respectively (see Table 1 for the primers used). The recipient plasmid was digested with NheI / SalI, the donor plasmid was digested with AvrII / SalI, and assembled by multiple rounds of ePathBricks (specific method reference: Xu, P., et al., 2012, ACS Synthetic Biology, 1(7): 256 -266), the above-mentioned pathway genes were assembled in the form of monocistrons...
Embodiment 2
[0035] Example 2: Preparation of whole-cell catalyst and optimization of D-psicose synthesis conditions
[0036] In order to increase the yield and conversion rate of D-psicose, the present invention further optimizes the conditions for preparing the whole cell catalyst and the reaction conditions for converting glycerol to synthesize D-psicose.
[0037] In the present invention, the preparation process of the whole cell catalyst has been optimized firstly: the concentration range of the inducer IPTG concentration is 0.001-0.5mM, the temperature range of the induced expression of the pathway gene is 15-30°C, and the time range of the induced expression of the pathway gene is 4-30°C. 20h. The results showed that the optimal concentration of inducer IPTG was 0.05mM ( Figure 4 A), the optimum induction temperature is 20°C ( Figure 4 B), the optimal induction time is 12h ( Figure 4 C). The present invention optimizes the reaction conditions for the conversion of glycerin to...
Embodiment 3
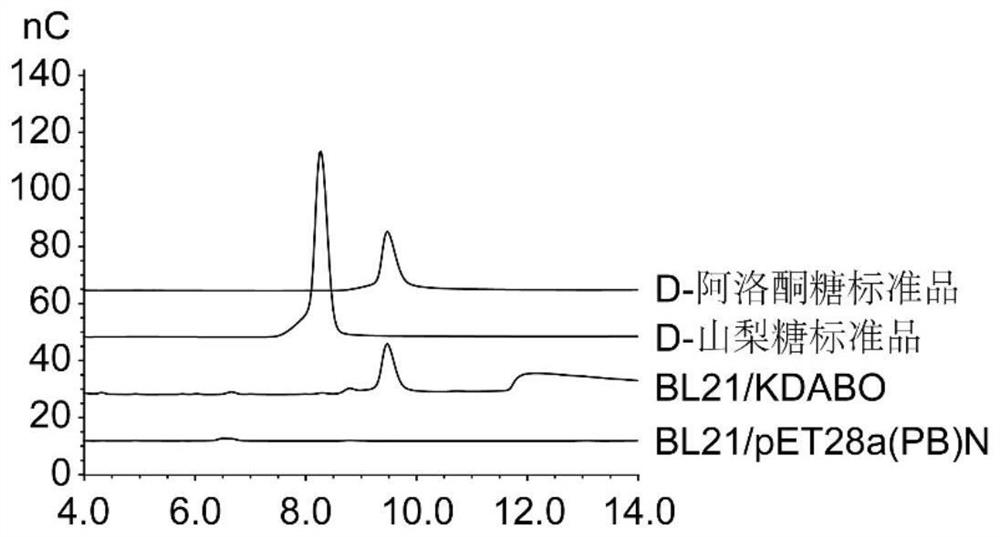
[0039] Example 3: Using a fermenter to amplify the reaction process to prepare D-psicose
[0040] In order to verify the feasibility of the D-psicose synthesis method described in the present invention in a small-scale production in a fermenter, on the basis of Example 2, the present invention uses a 5L fermenter to scale up the reaction system. After overnight activation of the recombinant Escherichia coli BL21 / KDABO, transfer it to a fermenter equipped with 3L LB medium at an inoculum size of 2% (v / v), at 37°C, 200rpm, and the condition that the ventilation rate is 4NI / min , cultured to OD 600 = 0.6-0.8. Add IPTG at a final concentration of 0.05mM, and induce for 12h at 20°C, 200rpm, and 4NI / min ventilation to express pathway genes. Under the conditions of 4°C and 5000rpm, centrifuge for 15 minutes to collect the bacteria, wash the bacteria with sterilized ultrapure water to remove the medium, and then resuspend the bacteria in 50mM phosphate buffer with pH 7.5, according ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com