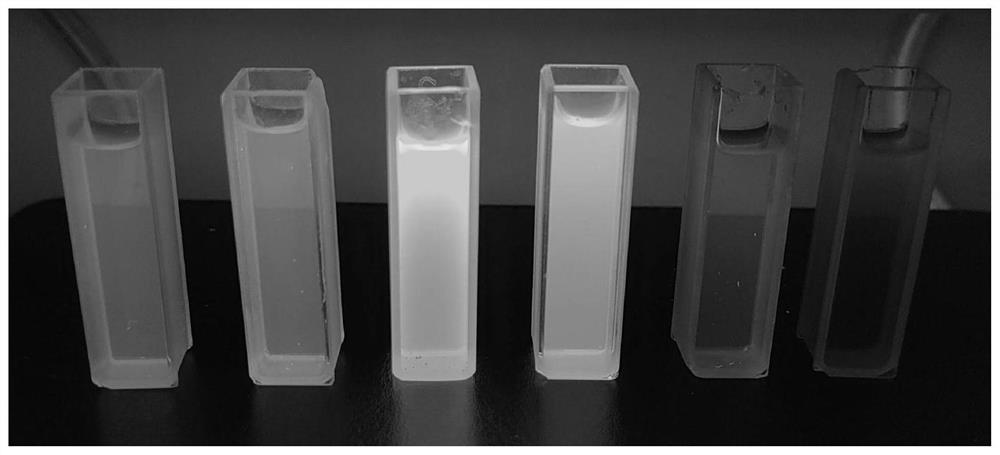
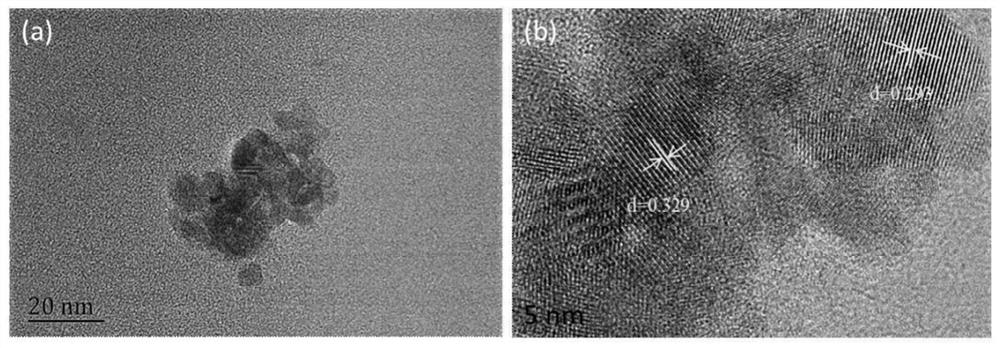
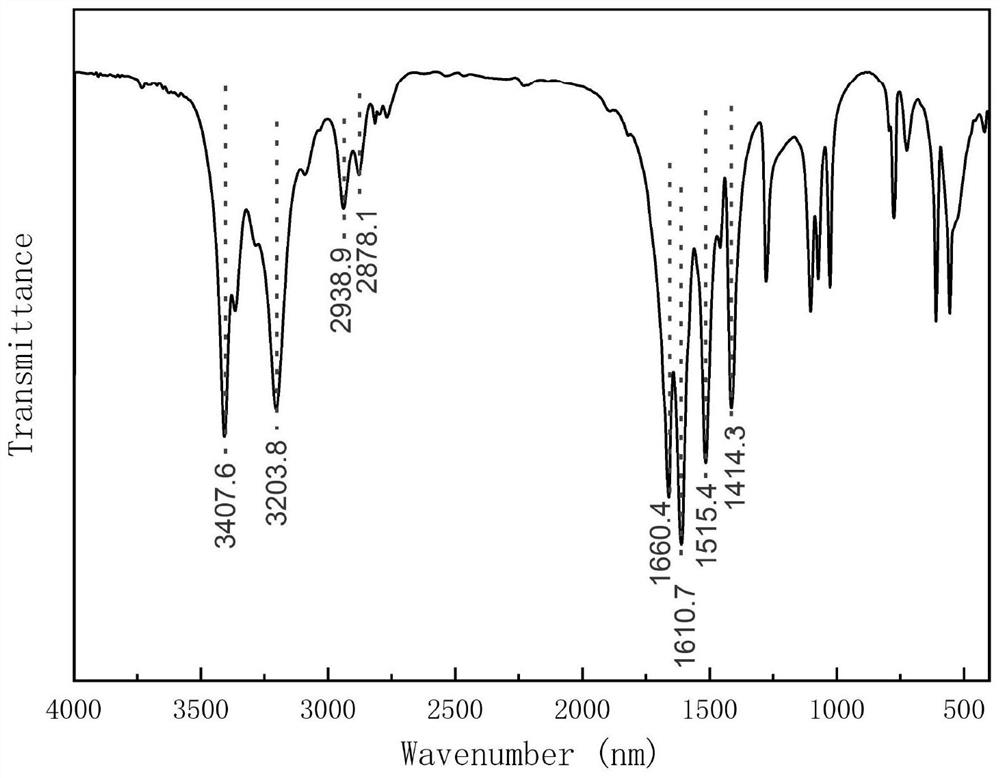
Preparation method and application of fluorescent carbon quantum dots
A technology of carbon quantum dots and fluorescence, which is applied in the field of preparation of fluorescent carbon quantum dots, can solve the problems of fluorescent carbon quantum dots limitations and inability to produce large quantities, and achieve the effect of promoting photoreaction and avoiding residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] Preparation of blue carbon quantum dots: Weigh 1.0g of citric acid and 1.2g of thiourea, then add 15mL of water into a sealed tube, and react at 140°C for 10h. After the reaction, cool to room temperature, and concentrate under reduced pressure to remove the solvent. The residue was purified by silica gel flash chromatography, concentrated and dried to obtain 1.8 g of light yellow solid powder.
[0040] Preparation of green carbon quantum dots: Weigh 1.5g of citric acid and 1.0g of urea, then add 15mL of formamide into a sealed tube, and react at 150°C for 12h. After the reaction, cool to room temperature, and concentrate under reduced pressure to remove the solvent. The product was purified by silica gel flash chromatography, concentrated and dried to obtain 1.2 g of yellow solid powder.
[0041] Preparation of yellow carbon quantum dots: Weigh 1.1g of p-phenylenediamine and 1.6g of urea, then add 15mL of dimethyl sulfoxide into a sealed tube, react at 160°C for 8h, af...
Embodiment 1
[0044]
[0045] Sequentially weigh p-methoxybenzenediazonium tetrafluoroborate 1a (0.3mmol), diboronic acid pinacol ester 2a (0.4mmol), B-CDots (10mg) into the photoreaction tube, under 0.3Mpa nitrogen atmosphere Add 5 mL of acetonitrile, and react at room temperature for 20 h under the light of 460 nm LEDs (10 W). After the reaction, extract with ethyl acetate and water, collect the organic phase, dry over anhydrous sodium sulfate, filter, remove volatile components under reduced pressure, and then use silica gel column chromatography to separate (eluent is petroleum ether (60-90°C) ) / ethyl acetate, v / v=20:1), the target product 3a was obtained as a colorless oil (59.7 mg, yield 85%). The target product was confirmed by NMR spectroscopy.
Embodiment 2
[0047]
[0048] Sequentially weigh benzenediazonium p-bromotetrafluoroborate 1b (0.3mmol), pinacol diborate 2a (0.45mmol), and B-CDots (15mg) into the photoreaction tube, and add them under an argon atmosphere of 0.3Mpa. 5 mL of toluene was reacted at room temperature for 18 h under the light of 495 nm LEDs (10 W). After the reaction, extract with ethyl acetate and water, collect the organic phase, dry over anhydrous sodium sulfate, filter, remove volatile components under reduced pressure, and then use silica gel column chromatography to separate (eluent is petroleum ether (60-90°C) ) / ethyl acetate, v / v=50:1), the target product 3b was obtained as a white solid (63.7 mg, yield 75%). The target product was confirmed by NMR spectroscopy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com