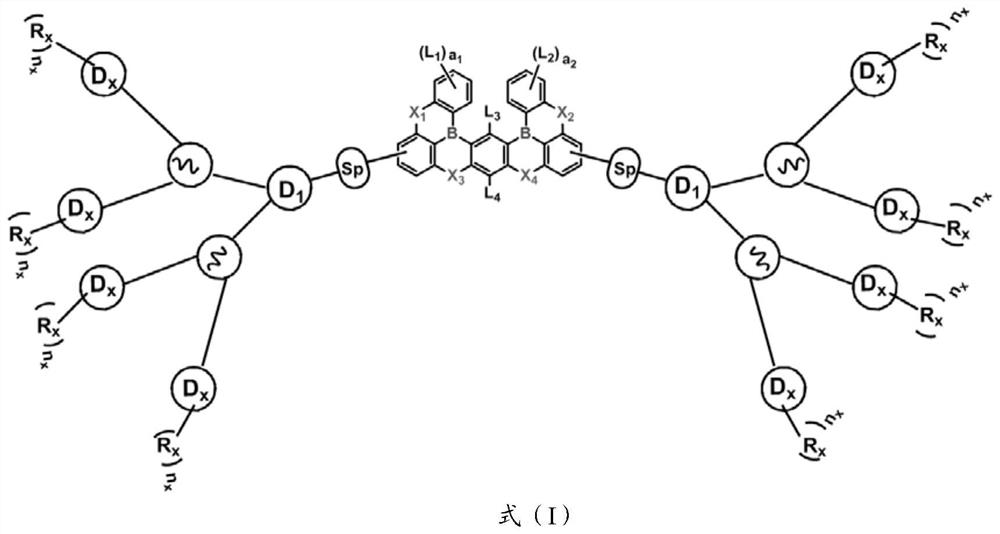
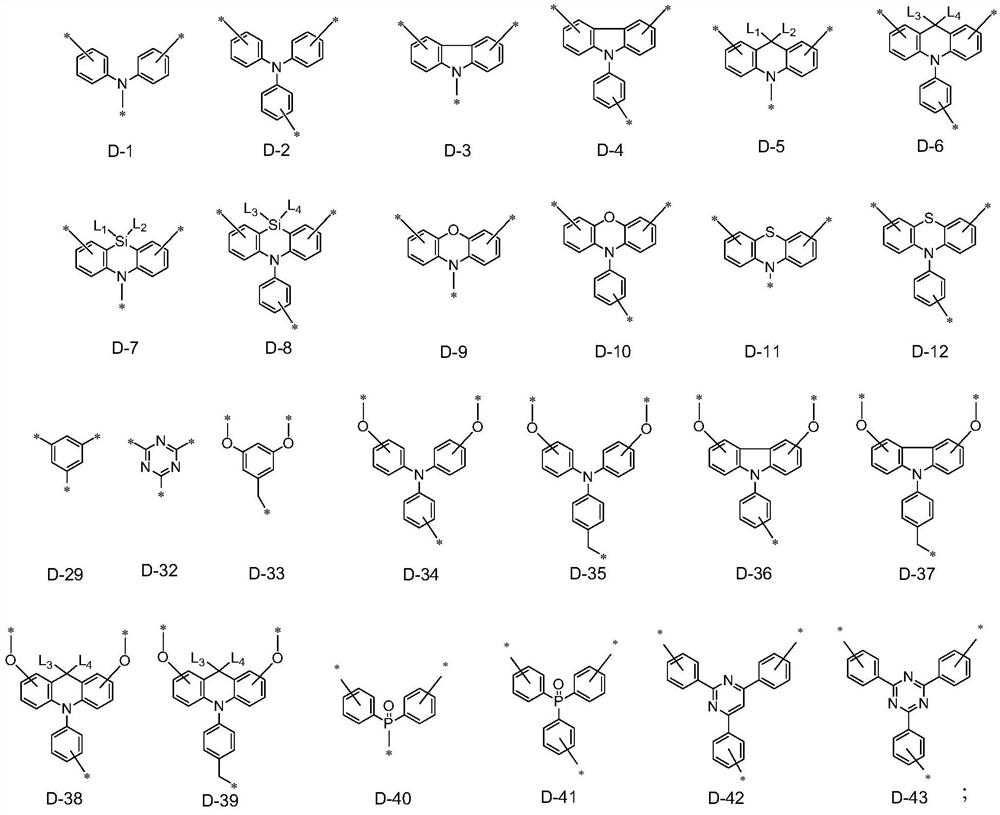
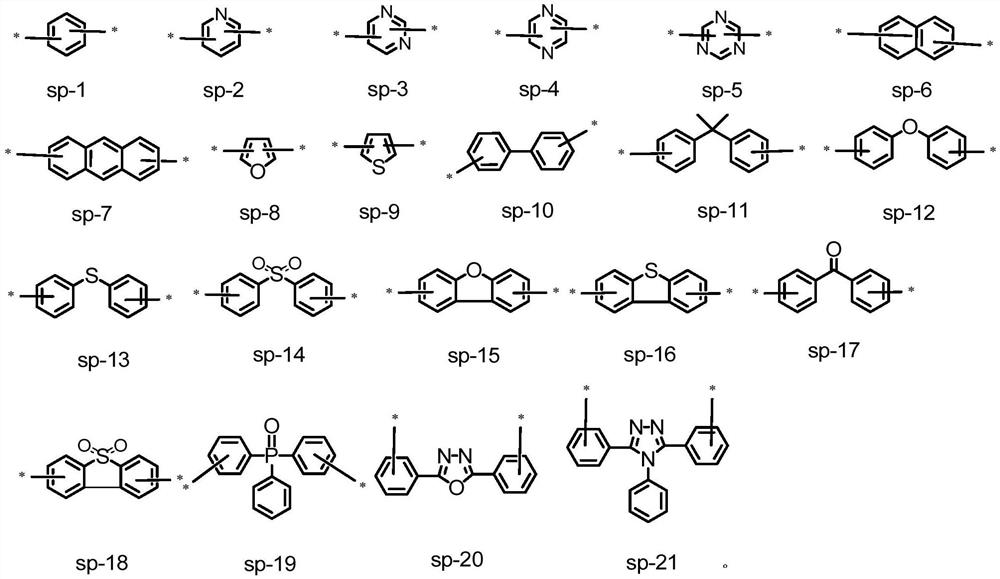
Organoboron fused ring compound containing dendritic structure and organic electroluminescent device
A molecular structure, organic boron technology, applied in the field of materials, can solve the problems of wide emission spectrum, low color purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] The present invention has no special limitation on the preparation method of the condensed ring compound, and a typical preparation process thereof is as follows:
[0058] The compound represented by formula (II) is reacted with the compound represented by formula (III) in a solvent to obtain the condensed ring compound represented by formula (I).
[0059]
[0060] Among them Lu 1 、Lu 2 、Lu 3 each independently selected from hydrogen, halogen, hydroxyl, mercapto, amino,
[0061] The present invention also provides an organic electroluminescent device, comprising an anode, a cathode, and an organic thin film layer between the anode and the cathode; the organic thin film layer includes the condensed ring compound shown in the above formula (I) .
[0062] The present invention has no special restrictions on the structure of the organic electroluminescent device, and conventional organic electroluminescent devices well known to those skilled in the art can be use...
Embodiment 1
[0078] The chemical structure and synthetic route of I-1 are as follows:
[0079]
[0080] Under an argon atmosphere, add 1-1((3,5-dichlorophenyl)-diphenylamine) (6.91 g, 22 mmol), N,N'-diphenyl-m-benzenediphenylamine to a 50 mL Schlenk bottle Amine (2.6g, 10mmol), Pd 2 (dba) 3 (0.46g, 0.5mmol), t-Bu 3 PHBF 4 (0.58g, 2mmol), t-BuONa (3.84g, 40mmol), then inject 20mL of toluene, and react at 110°C for 24 hours. Cool down to room temperature, add deionized water and 100 mL of dichloromethane for extraction, and wash with deionized water several times. The organic phase was separated, and the product 1-2 (4.5 g, yield: 55%) was obtained by column separation and desolventization. Elemental analysis: theoretical value C, 79.50; H, 4.94; N, 6.87; test value C, 79.47; H, 4.91; N, 6.89. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF (m / z)): theoretical value 814.3; experimental value 814.3 (M + ).
[0081] Under argon atmosphere, wei...
Embodiment 2
[0085] The chemical structure and synthetic route of I-2 are as follows:
[0086]
[0087] Under argon atmosphere, weigh 2-1((5-chloro-3-fluorophenyl)-diphenylamine) (2.98g, 10mmol), m-bromophenol (1.73g, 10mmol) and K 2 CO 3 (2.76g, 20mmol), take 40mL DMF and add in the bottle, heat up to 90°C, stir and react under argon protection for 8 hours, then cool to room temperature, pour the reaction solution into water after diluting the reaction solution with toluene, separate the organic phase, Anhydrous sodium sulfate was added to dry, and the obtained organic phase was filtered to remove the solvent, and the crude product was separated by column to obtain product 2-2 (2.9 g, yield: 65%). Elemental analysis: theoretical value C, 63.95; H, 3.80; N, 3.11; test value C, 63.91; H, 3.77; N, 3.14. Electrospray ionization mass spectrometry (ESI-MS): theoretical value 449.0; experimental value 449.0 (M+).
[0088] Under argon atmosphere, in a 50 mL Schlenk bottle, add 1-1 (3.1 g, 10 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com