Hyaluronic acid lyase as well as gene expression and application thereof
A technology of hyaluronic acid and lyase, applied in lyase, carbon-oxygen lyase, application, etc., can solve the problems of narrow temperature range and pH range of enzyme, uneven molecular weight of enzymatic hydrolysis products, cumbersome product separation steps, etc., to achieve The effect of wide temperature and pH range, small molecular weight and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A hyaluronan lyase, the amino acid sequence of which is shown in SEQ ID NO.1, the hyaluronan lyase is specifically obtained from a strain of Streptomyces alfalae screened in a laboratory.
[0033] Obtaining the coding gene of the above-mentioned hyaluronan lyase:
[0034] Using the genome of Streptomyces alfalae screened above as a template (DNA template), design upstream and downstream primers and predict the annealing temperature of the primers, add a BamHI restriction enzyme site and a protective base at the 5' end of the upstream primer, The 5′ end of the downstream primer introduces an XhoI restriction enzyme site and a protective base, and the primer sequence is as follows:
[0035] Sahyal-F: (BamHI) 5'-TTAAGAATTCGCCAGGGCCGCCGAGG-3' (SEQ ID NO.3);
[0036] Sahyal-R: (XhoI) 5'-CCGCTCGAGGCCCCGCAGGGTCACC-3' (SEQ ID NO. 4).
[0037] A large amount of amplification was performed using a PCR instrument. The DNA polymerase kit was purchased from Beijing Quanshijin Biot...
Embodiment 2
[0043] Construction of the recombinant expression vector of the above-mentioned hyaluronan lyase:
[0044] 1) Double enzyme digestion of the hyaluronan lyase-encoding gene and vector pET28a-SUMO amplified and purified in Example 1.
[0045] The coding gene of hyaluronan lyase and the vector pET28a-SUMO were respectively double-digested with restriction endonucleases BamHI and XhoI. The double-digestion system is shown in Table 3. Place the double enzyme digestion system in a 37°C water bath for 3 hours. After the end, add the above enzyme digestion system to 0.8% agarose gel for detection, and carry out the enzyme digestion gene fragment and pET28a-SUMO carrier band of the correct size. Cut gel recovery and purification.
[0046] Table 3 enzyme digestion system
[0047]
[0048] 2) Enzyme ligation of the digested gene fragment with the linearized pET28a-SUMO vector.
[0049]The purified enzyme-cut gene fragment and the linearized vector pET28a-SUMO were ligated by T4 DNA...
Embodiment 3
[0059] Acquisition of expression cells and expression of hyaluronan lyase.
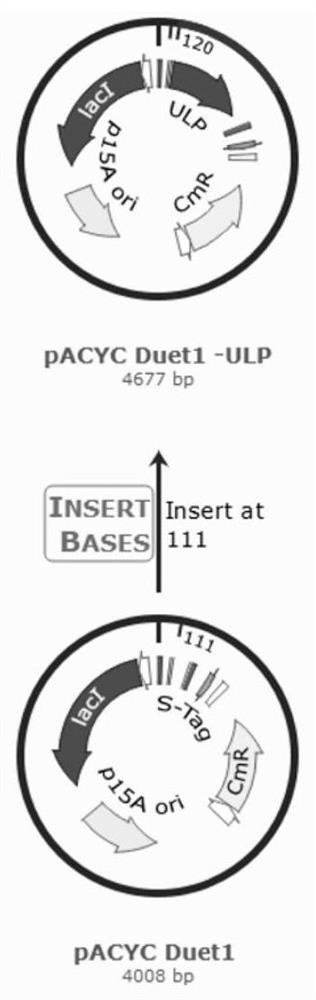
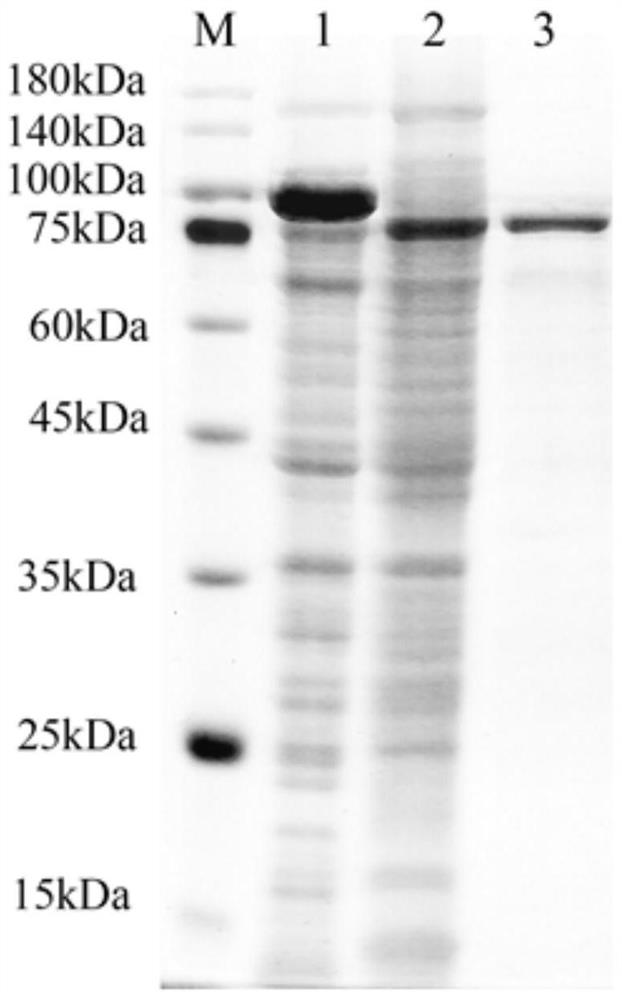
[0060] The plasmid pACYC Duet-1-ulp (used to express the SUMO protease Ubiquitin-Like protein-specific Protease Ulp without the His tag, which can specifically recognize and remove the SUMO tag, pACYC Duet-1-ulp was used for double-plasmid co-expression. The structure of the plasmid is shown in figure 1 As shown, it was obtained by inserting the gene sequence expressing Ulp in the pACYC Duet plasmid) and the recombinant plasmid pET28a-SUMO-Sahyal were simultaneously transformed into competent cells BL21 (DE3) by the heat shock method, and 400 μL of anti-LB liquid medium was added, Incubate at 37°C, 180rpm for 60min. Take 80 μL of the above-mentioned bacterial solution and evenly spread it on a double-antibody (containing both kan and Cm resistance) solid LB plate, and culture it upside down at 37°C overnight. Pick a single colony in 2 mL of LB liquid medium (both containing kan and Cm resistance). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com