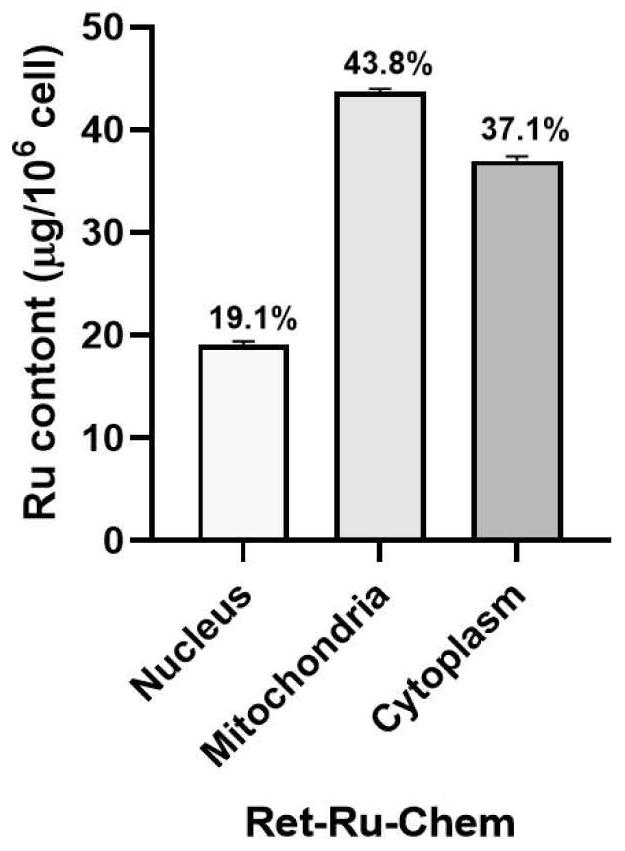
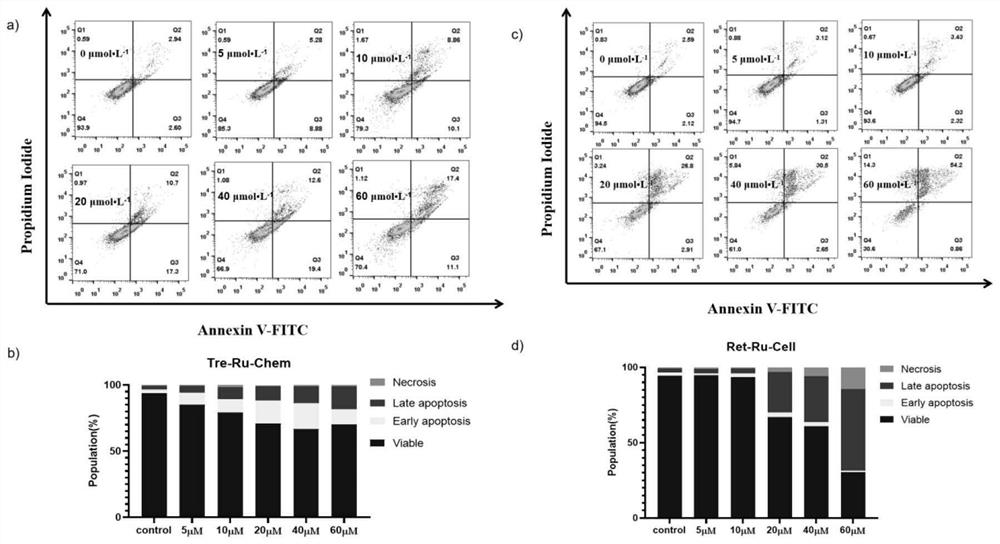
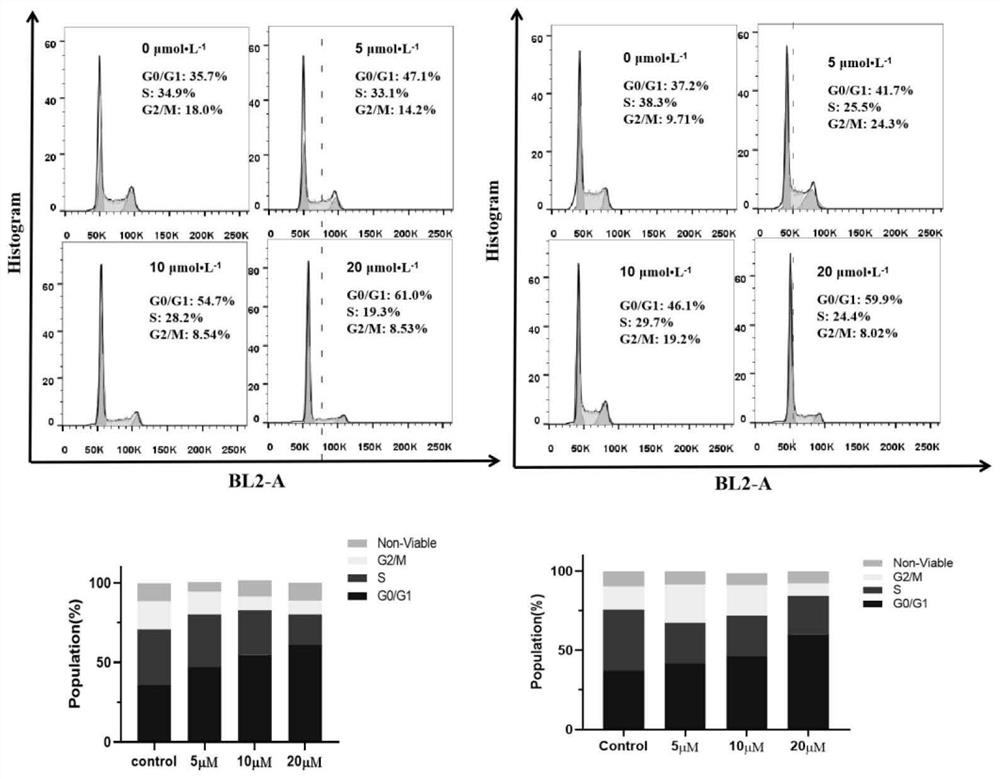
All-trans retinoic acid-aryl metal complex as well as preparation method and application thereof
A technology of all-trans retinoic acid and metal complexes, applied in the field of biochemistry, can solve the problems of difficult metabolism, large toxic and side effects, and achieve the effects of high cellular uptake, low drug resistance, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] The preparation method of the metal complex Ret-Ru of the present invention comprises the following steps:
[0037] Step 1, under argon atmosphere, dissolve retinoic acid, N-hydroxybenzotriazole and N,N-diisopropylethylamine in DMF, react for 5min at a certain temperature, and mix a certain amount of 1-(3 -Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride was dissolved in DMF and added dropwise to the reaction mixture. After the mixture was reacted at 0°C for a period of time, propargylamine was added dropwise to the mixed solution. The reaction mixture was allowed to warm to room temperature overnight. After stirring overnight, the organic solvent was removed and the resulting residue was dissolved in ethyl acetate, followed by 5% NaHCO 3(3 x 20 mL) and 0.5 mol / L HCl (3 x 20 mL). Leave the aqueous hydrochloric acid phase, by adding Na 2 CO 3 The pH of the aqueous solution was adjusted to around 8.0 and extracted with dichloromethane (3 x 20 mL) to...
Embodiment 2
[0044]
[0045] The preparation method of the metal complex Ret-Ir of the present invention comprises the following steps:
[0046] Step 1, under argon atmosphere, dichloro(pentamethylcyclopentadienyl)iridium(III) dimer and 4-azidomethyl-4'-methyl-2,2'-linked Pyridine (Example 1) was dissolved in methanol solution, reacted at room temperature for 48 hours, the solvent was removed by rotary evaporation, 10 times equivalent saturated methanol solution of ammonium hexafluorophosphate was added, and column chromatography was performed to obtain a bright yellow powder product with a yield of 86 %, named Ir-N 3 . 1 H NMR (400MHz, DMSO-d 6 )δ8.95(d, J=5.9Hz, 1H), 8.82(d, J=5.8Hz, 1H), 8.73(d, J=5.6Hz, 2H), 7.79(d, J=5.8Hz, 1H) , 7.71(s, 1H), 4.95(s, 2H), 2.64(s, 3H), 1.65(s, 15H).
[0047] Step 2, under argon atmosphere, mix 0.05mmol Ret-alkyne, 0.15mmol Ir-N prepared above 3 , 0.01mmol of copper sulfate pentahydrate and 0.02mmol of sodium ascorbate were dissolved in DMF and ...
Embodiment 3
[0049]
[0050] The preparation method of metal complex Per-Ru of the present invention comprises the following steps:
[0051] Step 1, under argon atmosphere, dissolve 1 mmol of perillyl alcohol, 1 mmol of bromopropyne and 2 mmol of potassium carbonate in DMF, and react at 80 °C for 10-16 h. After the reaction, the reaction solution is washed with an equal volume of ethyl acetate. It was diluted with water solvent, the organic layer was separated, and the aqueous phase was extracted with ethyl acetate. The organic layers were combined and dried over anhydrous sodium sulfate, the solvent was removed, and the pure product was obtained by column chromatography with a yield of 56%. Named Per-alkyne. 1 H NMR (400MHz, DMSO-d 6)δ5.48(m,1H),5.11(d,J=3.2Hz,1H),4.92(d,J=4.6Hz,1H),4.15(m,2H),4.04(m,2H),3.32( s, 1H), 2.33(dd, J=2.2Hz, 1H), 2.09 -7.61(d, J=5.8Hz, 2H), 1.99(dd, J=12.1Hz, 2H), 1.79(s, 3H), 1.77 (dd, J=2.2Hz, 2H).
[0052] Step 2, under argon atmosphere, mix 0.05mmol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com