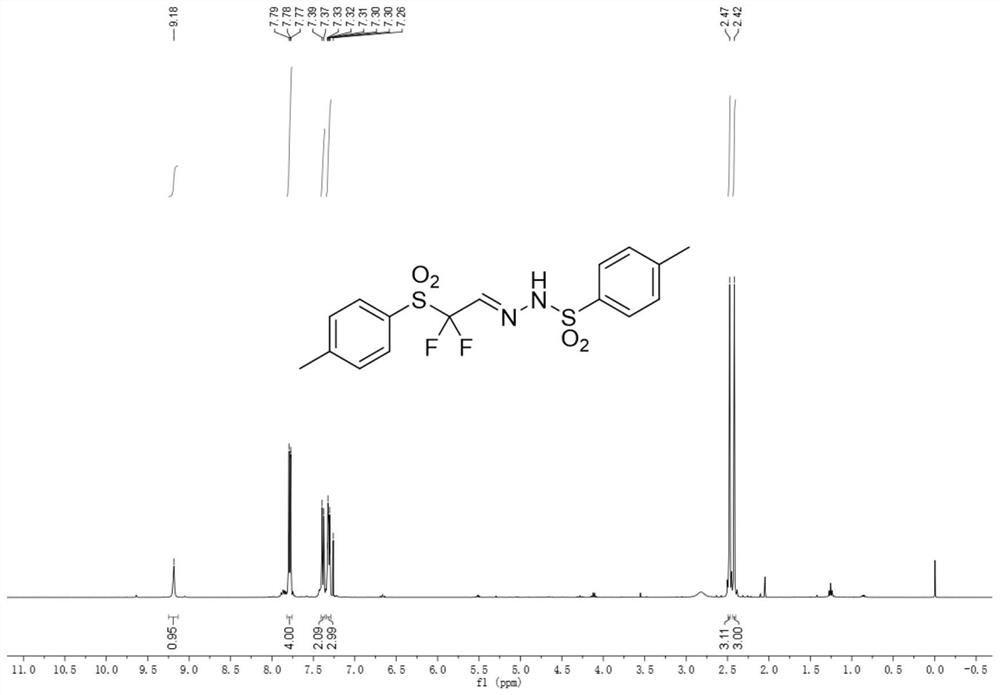
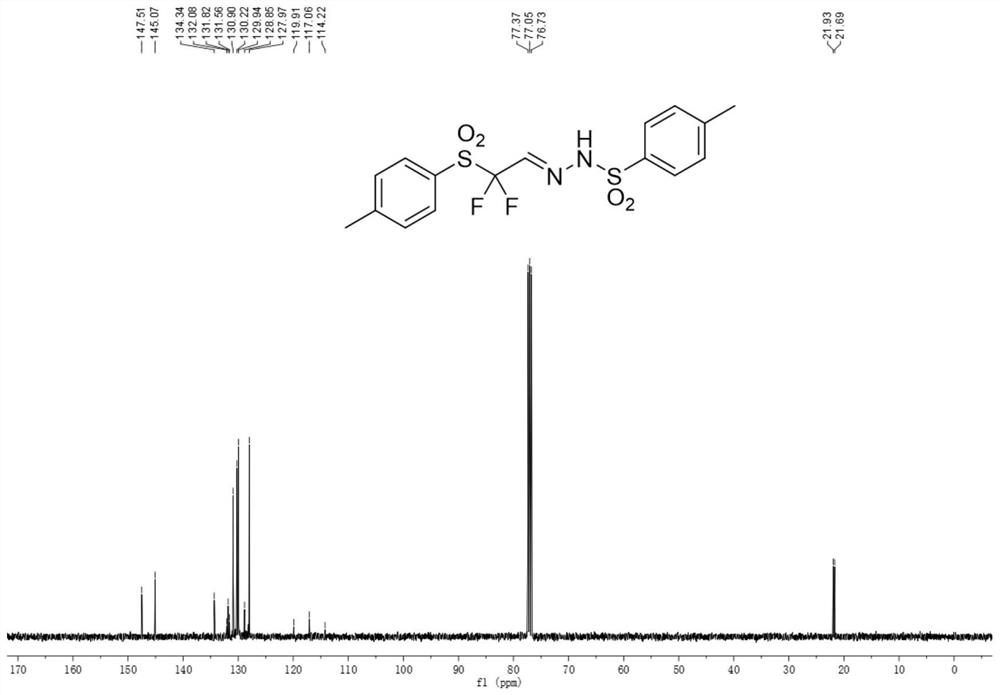
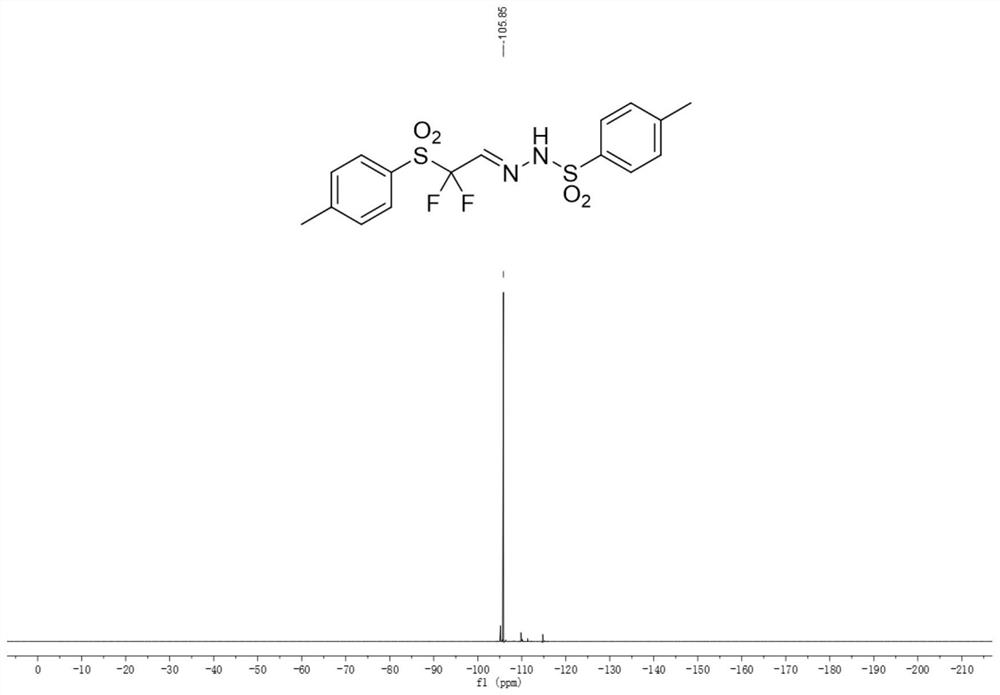
Aromatic sulfonyl modified difluoromethyl reaction block and synthesis method thereof
A technology of difluoromethyl reaction and aromatic sulfonyl group, which is applied in the field of synthesis and development of difluoromethyl reaction building blocks, can solve the problems of unstable reagent properties, harsh reaction conditions, and difficult availability of raw materials, etc., and achieve good universality Sex, stable and safe, easy to store
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
[0049] (4-1)
[0050] 1) Dissolve 20 mmol of p-methylthiophenol in 20 mL of dimethyl sulfoxide, add 2.0 equivalents of sodium hydride (40 mmol), stir at room temperature for 1 hour, and then add 48 mmol of ethyl bromodifluoroacetate , stirred at room temperature for 12 hours, then washed, extracted, concentrated, and directly put into the next reaction without further purification; 2) The above product was dissolved in 20 mL of ethanol, and then 2.0 equivalent of sodium borohydride (40 mmol) was added, at 0 °C The reaction was carried out for 0.5 hours, washed, extracted and concentrated to obtain primary alcohol derivatives substituted with difluorosulfide, which was directly put into the next reaction without purification; 3) The above product was dissolved in a mixed solution of 40 mL of water and acetic acid (volume ratio 1 : 1), add 4.0 equivalents of hydrogen peroxide, heat under reflux for 2 hours, wash, extract, and concentrate to obtain a crude product, wh...
Embodiment 2
[0053]
[0054] (4-2)
[0055] 1) Dissolve 20 mmol of 2,4-dimethylthiophenol in 40 mL of dimethyl sulfoxide, add 1.5 equivalents of sodium hydride (30 mmol), stir at room temperature for 1 hour, and then add 50 mmol of bromine Ethyl difluoroacetate was stirred at room temperature for 12 hours, followed by washing, extraction, and concentration. It was directly used in the next reaction without further purification; 2) The above product was dissolved in 20 mL of ethanol, and then 2.0 equivalent of sodium borohydride (40 mmol) was added. , reacted at 0 °C for 0.5 hours, washed, extracted and concentrated to obtain difluorosulfide-substituted primary alcohol derivatives, which were directly put into the next step without purification; 3) The above product was dissolved in a mixture of 40 mL of water and acetic acid To the solution (volume ratio 1:1), add 4.0 equivalents of hydrogen peroxide, heat under reflux for 2 hours, wash, extract, and concentrate to obtain a crude produc...
Embodiment 3
[0058]
[0059] (4-3)
[0060] 1) Dissolve 20 mmol of 3-bromothiophenol in 40 mL of dimethyl sulfoxide, add 2.0 equivalents of sodium hydride (40 mmol), stir at room temperature for 1 hour, and then add 60 mmol of ethyl bromodifluoroacetate , stirred at room temperature for 12 hours, then washed, extracted, concentrated, and directly put into the next reaction without further purification; 2) The above product was dissolved in 20 mL of ethanol, and then 3.0 equivalent of sodium borohydride (60 mmol) was added, and the reaction was carried out at 0 °C. The reaction was carried out for 0.5 hours, washed, extracted and concentrated to obtain primary alcohol derivatives substituted with difluorosulfide, which was directly put into the next reaction without purification; 3) The above product was dissolved in a mixed solution of 40 mL of water and acetic acid (volume ratio 1 : 1), add 4.0 equivalents of hydrogen peroxide, heat under reflux for 2 hours, wash, extract, and concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com