PH reversibly activated near-infrared two-region aggregation-induced emission type I photosensitizer and application thereof
A technology of aggregation-induced luminescence and photosensitizers, applied in the field of photosensitizers, can solve the problems of large side effects and poor targeting, and achieve the effects of enhanced active oxygen generation capacity, good light and heat, and excellent tumor cell targeting ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
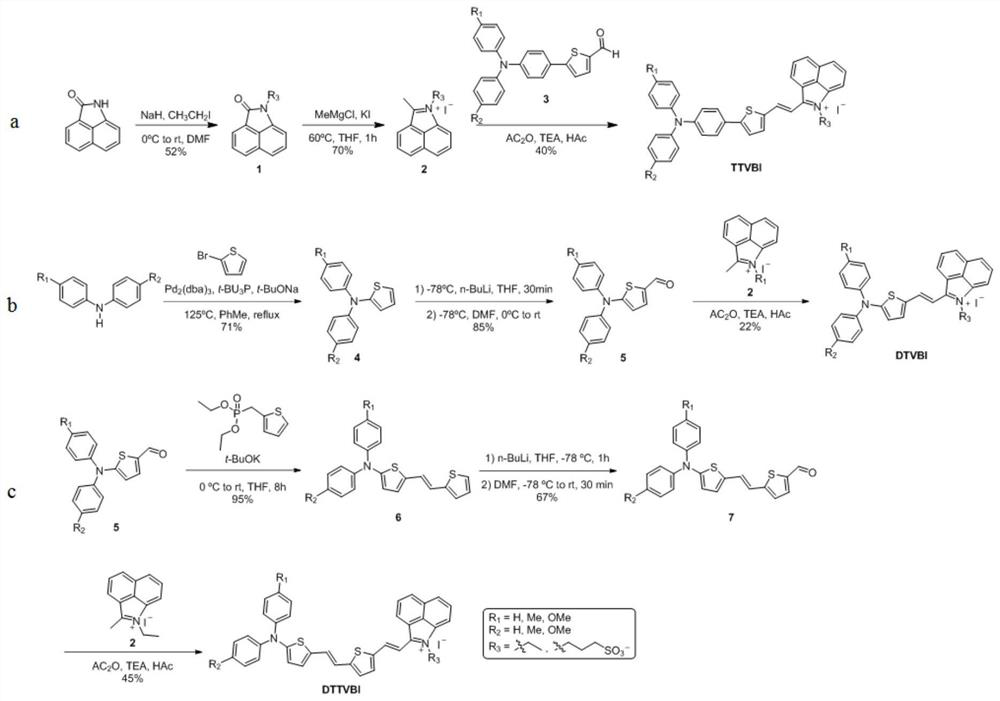
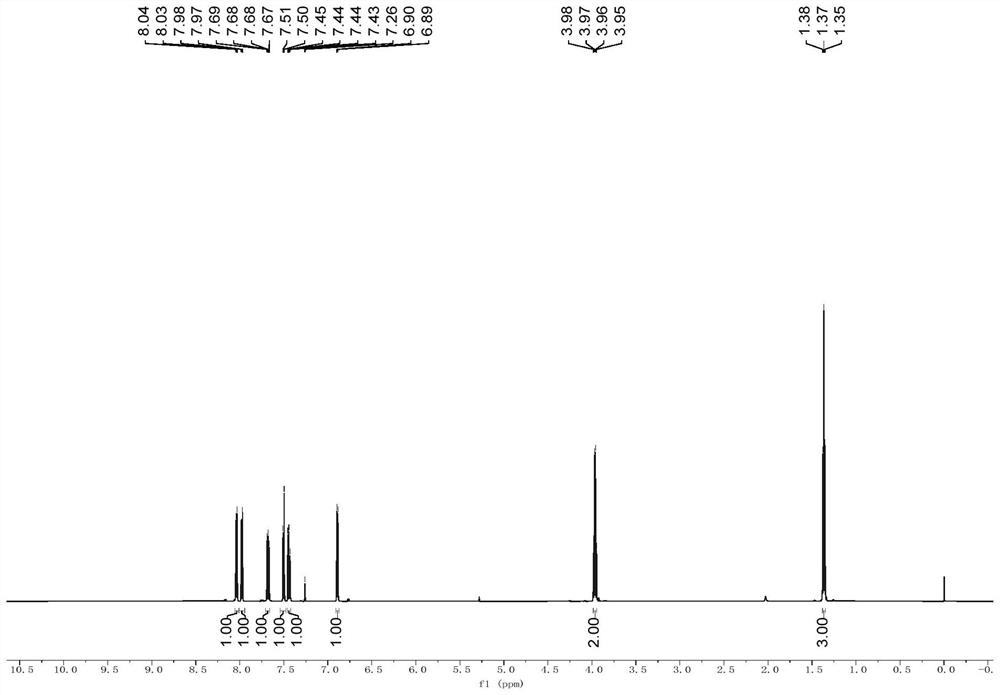
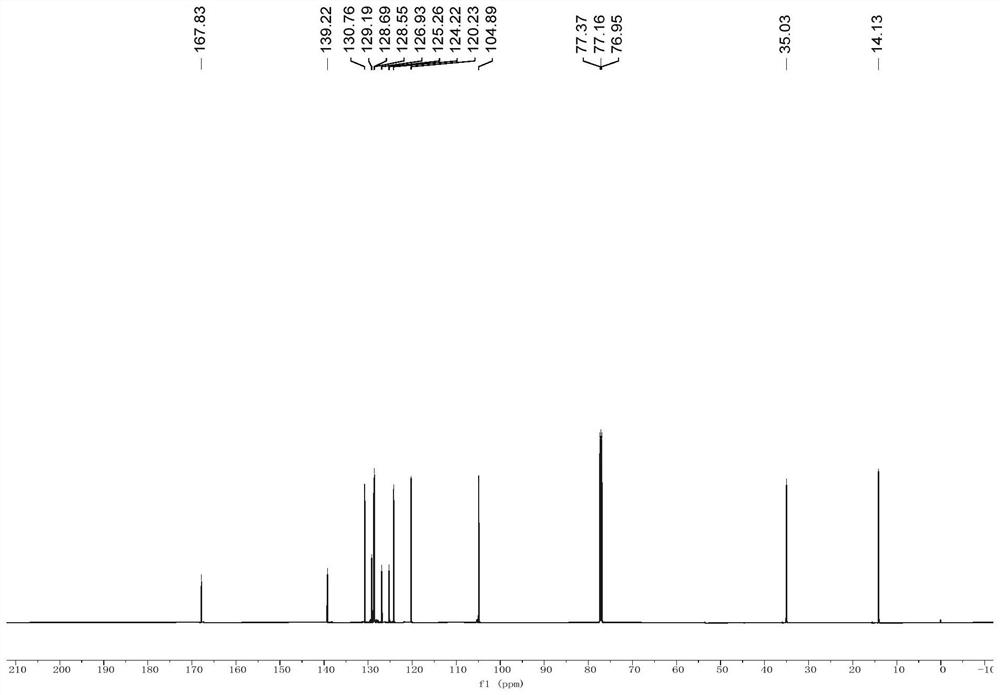
[0056] like figure 1 shown in a, in N 2 under the protection of The reaction was continuously stirred for 3 h, and extracted with ethyl acetate after the reaction was completed. The organic phases were combined and dried with anhydrous sodium sulfate. After concentration under reduced pressure, the residue obtained was washed with petroleum ether / ethyl acetate (100:1→10:1). The removal agent was purified by silica gel column chromatography to obtain 1.20 g of yellow powdery solid compound 1 in a yield of 52%. The hydrogen nuclear magnetic resonance spectrum of the compound 1 in deuterated chloroform is as follows figure 2 As shown, the carbon nuclear magnetic resonance spectrum of the compound 1 in deuterated chloroform is as follows image 3 shown, where, 1 H NMR (600MHz, CDCl 3 )δ8.04(d,J=6.6Hz,1H),7.98(d,J=8.4Hz,1H),7.68(dd,J 1 =7.8Hz,J 2 =6.6Hz,1H),7.50(d,J=8.4Hz,1H),7.44(dd,J 1 =8.4Hz,J 2 =7.2Hz,1H),6.89(d,J=6.6Hz,1H),3.97(q,J=7.2Hz,2H),1.37(t,J=7.2Hz,3H). 13 C...
Embodiment 2
[0058] like figure 1 shown in a, in N 2 Under the protection of , 1.20g of compound 1 was dissolved in 25ml of dry tetrahydrofuran solution, and then 2.43ml of 3.0M methylmagnesium chloride solution was added dropwise to the above reaction solution. After dropping, the reaction was stirred at 60 °C for 1 h, and the reaction solution was cooled After reaching room temperature, 12.16 ml of 2M hydrochloric acid solution was added, and the tetrahydrofuran was removed by concentration under reduced pressure, then 6.08 ml of 1M potassium iodide solution was added, and a large amount of red precipitates were precipitated by continuing the stirring reaction for 30 min. After filtration, the obtained solid was washed with water and ethyl acetate successively, and dried to obtain 1.52 g of crude compound 2 as a red solid powder with a yield of 70%. The hydrogen nuclear magnetic resonance spectrum of the compound 2 in dimethyl sulfoxide is as follows Figure 4 shown, where, 1 H NMR (6...
Embodiment 3
[0060] like figure 1 shown in b, in N 2 Under the protection of the 2 (dba) 3 and 274 mg of tri-tert-butylphosphine tetrafluoroborate. Then the reaction solution was heated to 120 °C in an oil bath for 16 h. After cooling to room temperature, the solvent was concentrated under reduced pressure to remove the solvent, and the obtained residue was purified by silica gel column chromatography using petroleum ether / ethyl acetate (200:1→50:1) as eluent to obtain 4.20g of yellow powdery solid compound 4, Yield 71%. The hydrogen nuclear magnetic resonance spectrum of the compound 4 in deuterated chloroform is as follows Figure 5 As shown, the carbon nuclear magnetic resonance spectrum of the compound 4 in deuterated chloroform is as follows Image 6 shown, where, 1 H NMR (400MHz, Methylene Chloride-d 2 )δ7.29(d,J=5.2Hz,2H),7.26(d,J=5.2Hz,2H),7.14(dd,J 1 =8.8Hz,J 2 =1.2Hz,4H),7.05–7.01(m,3H),6.91(dd,J 1 =5.6Hz,J 2 =5.2Hz,1H),6.74(dd,J 1 =1.2Hz,J 2 =3.6Hz,1H). 13 CNMR (1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com