Preparation method of normal value quality control product for blood coagulation and platelet function analyzer
A technology for functional analysis and platelets, applied in the preparation of test samples, analytical materials, instruments, etc., can solve problems such as complex technical solutions, matrix effects, and difficult production control, and achieve simple preparation processes, good performance, and stability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of normal value quality control product
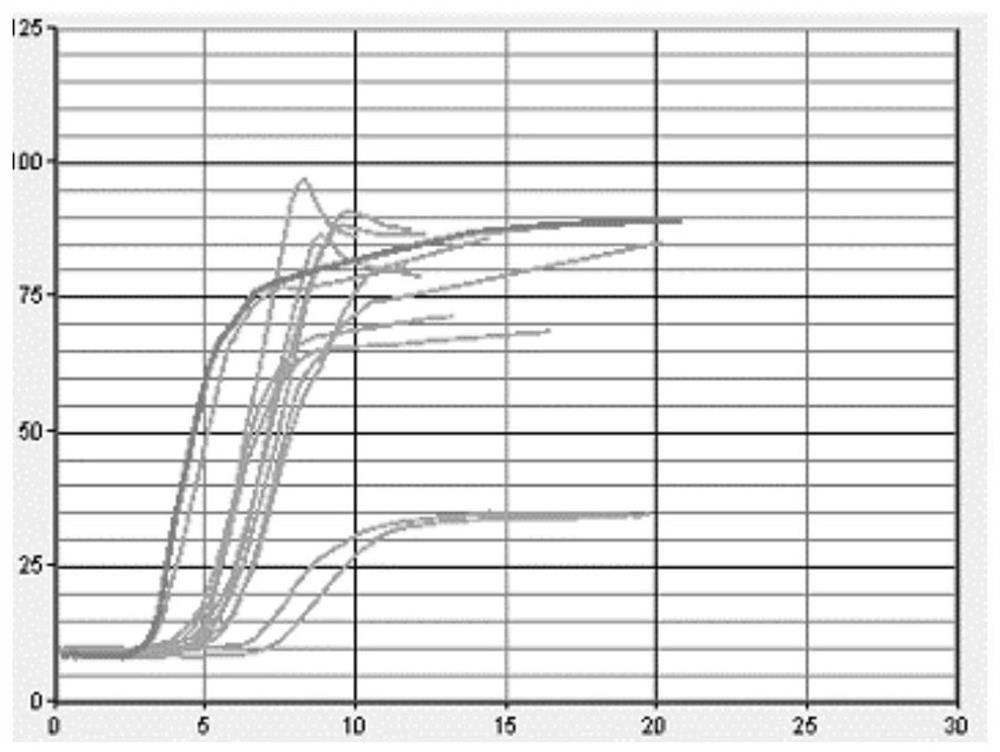
[0048] Step 1: Using sodium citrate anticoagulant human plasma as the matrix fluid, the matrix fluid was tested with a coagulation and platelet function analyzer produced by Sienco. The whole blood coagulation activation time (ACT) was in the range of 100-240 seconds, and the coagulation rate ( CR) in the range of 10-36 with platelet function (PF) >1. Matrix fluid test results see figure 1 .
[0049]Step 2: Mix the matrix solution with pH7.2 100mM Tris hydrochloride buffer at a ratio of 9:1, stir for 10 minutes, add 1‰ sodium azide, 2% trehalose, 2% white Egg white, stir for 30 minutes, and after it is completely dissolved, divide it into 1ml / bottle, freeze-dry it with a vacuum freeze dryer, take it out, cap the bottle, etc., and store it at 2-8°C. The test results of the quality control products after lyophilization are shown in figure 2 .
Embodiment 2
[0050] The preparation of embodiment 2 normal value quality control substance
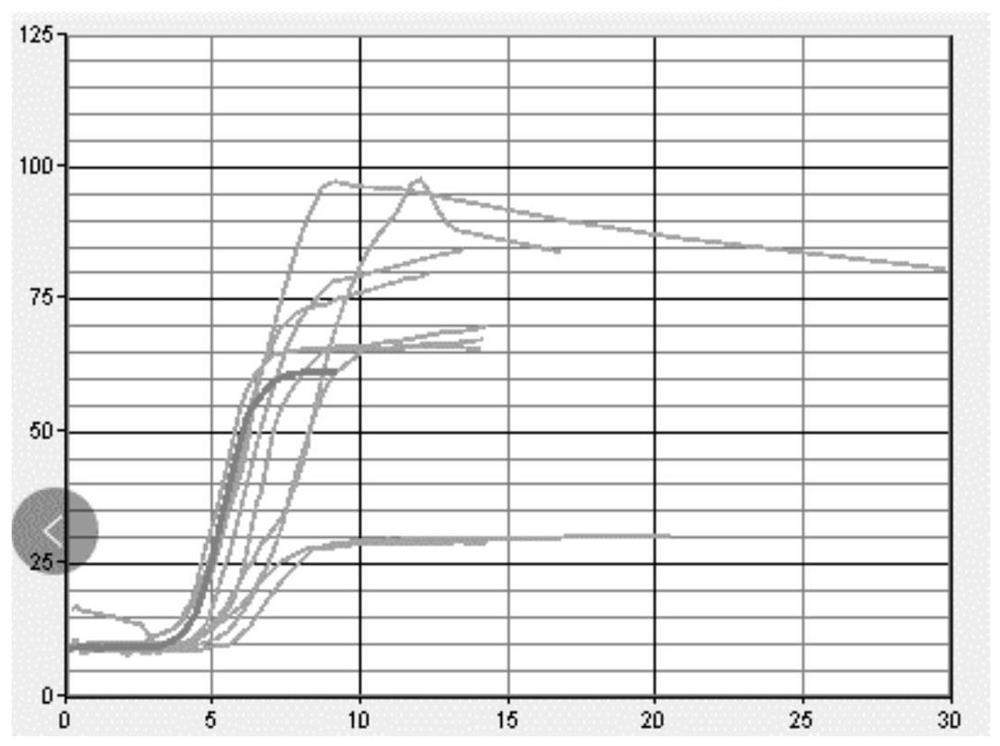
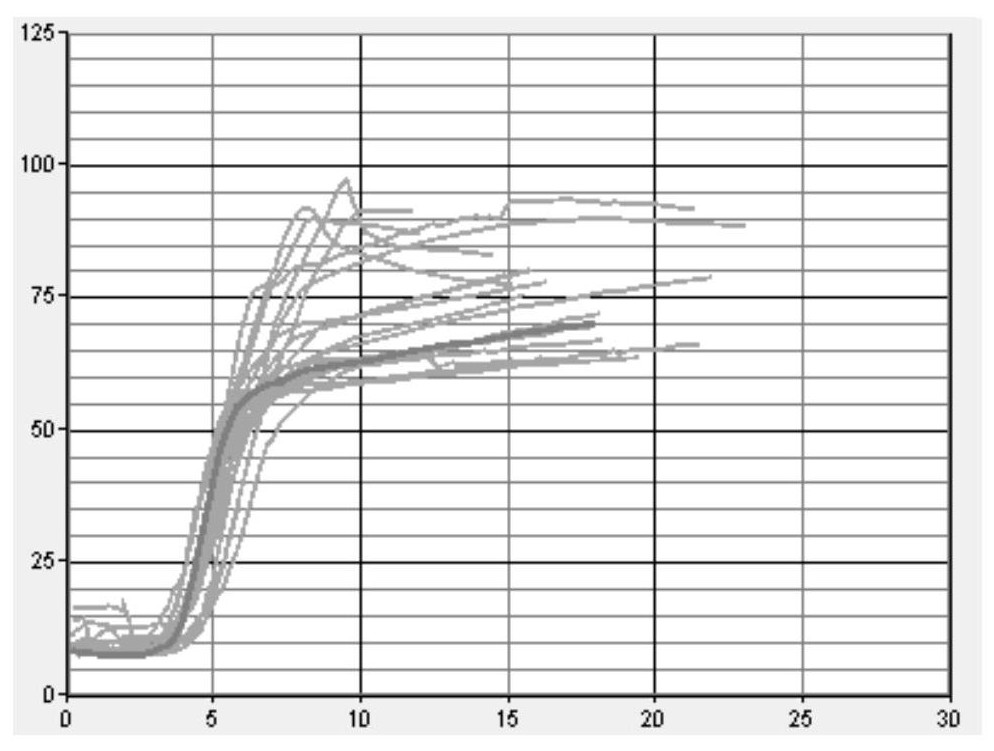
[0051] Step 1: Using sodium citrate anticoagulant human plasma as the matrix fluid, the matrix fluid was tested with a coagulation and platelet function analyzer produced by Sienco. The whole blood coagulation activation time (ACT) was in the range of 100-240 seconds, and the coagulation rate ( CR) in the range of 10-36 and platelet function (PF) image 3 . A 0.2 g / mL phosphatidylserine solution (0.2 g phosphatidylserine in 1 ml chloroform) was added to adjust PF to greater than 1. After adding phospholipid solution for adjustment, see the test results Figure 4 .
[0052] Step 2: Mix the matrix solution with pH7.2 100mM Tris hydrochloride buffer at a ratio of 9:1, stir for 10 minutes, add 1‰ sodium azide, 2% trehalose, 2% white Egg white, stir for 30 minutes, and after it is completely dissolved, divide it into 1ml / bottle, freeze-dry it with a vacuum freeze dryer, take it out, cap the bottle, e...
Embodiment 3
[0053] Example 3 Performance measurement of the quality control product prepared by the present invention
[0054] The quality control products for normal level coagulation and platelet function analyzers prepared in Examples 1 and 2 were successively investigated for bottle opening stability at 4°C, accelerated stability at 37°C and repeated freezing and thawing. The specific measurement data are shown in the following table:
[0055]
[0056]
[0057]
[0058]
[0059]
[0060] Conclusion: The normal value control products prepared in Example 1 and Example 2 are stable in nature, and can be stable for 2 days after opening at 4 °C; the relative deviations of ACT, CR and PF measured at 37 °C for 14 days are all within 15%; repeated freezing and thawing 5 times The relative deviations of the determinations were all within 15%; the coefficients of variation of the uniformity results were all less than 10%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com