Pyrene fused ring molecule based on bridged tripolyindole structure and electroluminescent device
A tripolybenzdole and pyrene technology, applied in the field of organic optoelectronics, can solve the problems of poor color purity, unfavorable high-end display applications, etc., and achieve the effects of good solubility, inhibition of non-radiative transition, and reduction of fluorescence quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention has no special limitation on the preparation method of the electroluminescent device, which can be carried out according to the following methods: forming an anode on the substrate; forming one or more organic thin film layers on the anode, including a layer of a light-emitting layer; forming a cathode on the organic thin film layer;
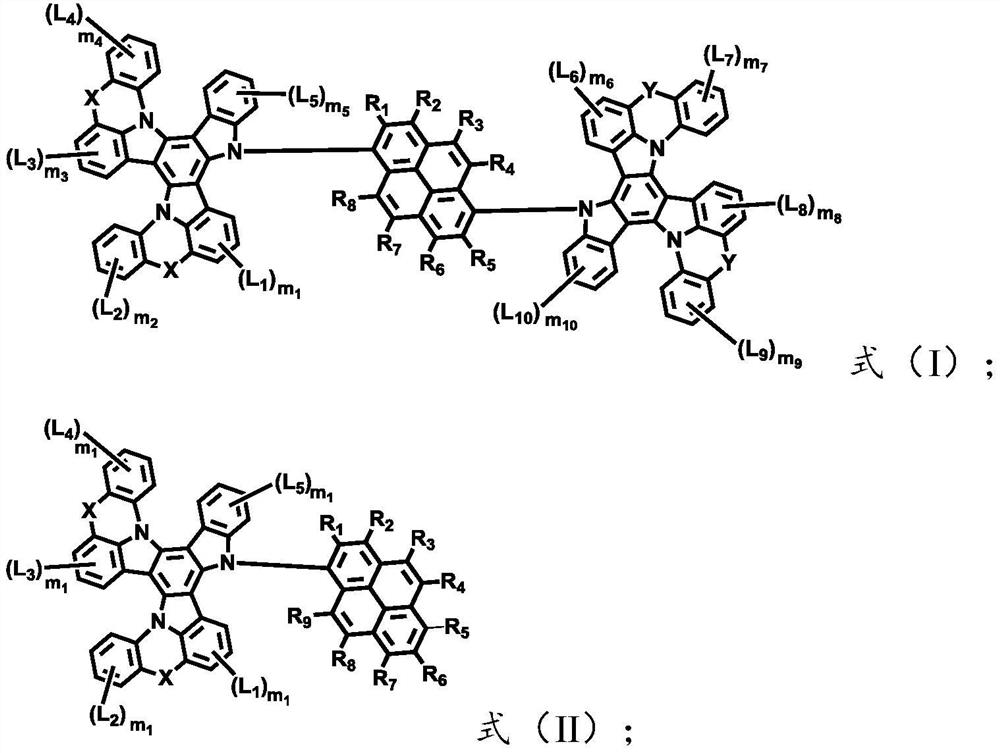
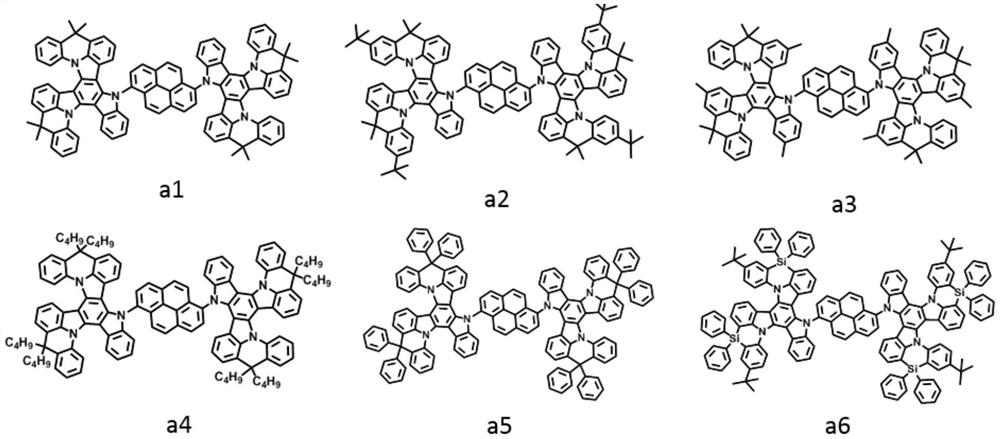
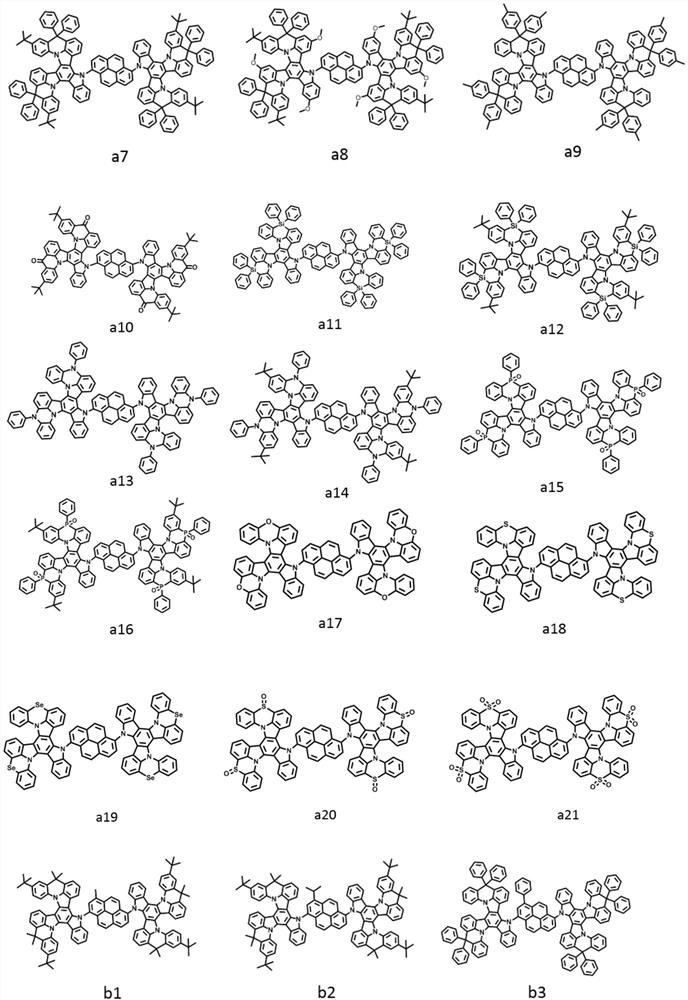
[0043] The light-emitting layer includes one or more pyrene-based fused ring molecules represented by formula (I) and / or formula (II) based on a bridged tripolybenzazole structure.
[0044] The present invention can correspond to the structures and materials of the electroluminescent device in the above preparation method, and the corresponding preferred principles, and the corresponding materials and structures of the aforementioned electroluminescent device, and the corresponding preferred principles, which will not be repeated here. One more elaboration.
[0045] In the present invention, an anode is first formed...
Embodiment 1
[0049]
[0050]1.1 Under argon atmosphere, weigh m-1 (3.45g, 10mmol), cuprous iodide, (190mg, 1mmol), copper powder (2.5g, 40mmol) and potassium carbonate (5.5g, in a 100mL two-necked flask). 40mmol), add 50mL of o-dichlorobenzene (o-DCB), 8ml of methyl o-iodobenzoate (50mmol), warm up to 220 ° C, stir under argon protection for 50 hours, then cool to room temperature, add dichloromethane and After extraction with water, the organic phase was separated, dried by adding anhydrous sodium sulfate, the solvent was removed from the organic phase obtained by filtration, and the product m-2 (800 mg, yield: 13%) was obtained by column separation.
[0051] Elemental Analysis Structure (C 40 H 27 N 3 O 4 ): theoretical C, 78.29; H, 4.43; N, 6.85; tested C, 78.30; H, 4.40; N, 6.80. Matrix-assisted laser desorption-time-of-flight mass spectrometry (MALDI-TOF-MS) theoretical value 613.20; experimental value 613.2 (M + ).
[0052] 1.2 Under an argon atmosphere, m-2 (1.2 g, 2 mmol) ...
Embodiment 2
[0059]
[0060] 2.1 Under argon atmosphere, in a 100mL two-necked flask, weigh m-1 (3.45g, 10mmol), 4-tert-butyl-2-bromoiodobenzene (16.9g, 50mmol), cuprous iodide, (190mg, 1mmol), copper powder (2.5g, 40mmol) and potassium carbonate (5.5g, 40mmol), add 50mL o-dichlorobenzene (o-DCB), warm up to 220°C, stir for 50 hours under argon protection, and then cool to At room temperature, dichloromethane and water were added for extraction, the organic phase was separated, dried by adding anhydrous sodium sulfate, the solvent was removed from the organic phase obtained by filtration, and the product m-6 (900 mg, yield: 12%) was obtained by column separation.
[0061] Elemental Analysis Structure (C 44 H 37 Br 2 N 3 ): theoretical C, 68.85; H, 4.86; N, 5.47; tested C, 68.41; H, 4.80; N, 5.60. Matrix-assisted laser desorption-time-of-flight mass spectrometry (MALDI-TOF-MS) theoretical value 765.14; experimental value 765.1 (M + ).
[0062] 2.2 Under an argon atmosphere, m-6 (76...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com