Alkaline functionalized porous polyion liquid material as well as preparation method and application thereof
A technology of polyionic liquid and ionic liquid, which is applied in the field of alkaline functionalized porous polyionic liquid material and its preparation, can solve the problems of low adsorption capacity, poor mass transfer effect of non-porous materials and limit the application, so as to improve the adsorption capacity, The effect of excellent thermal stability and high efficiency separation selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
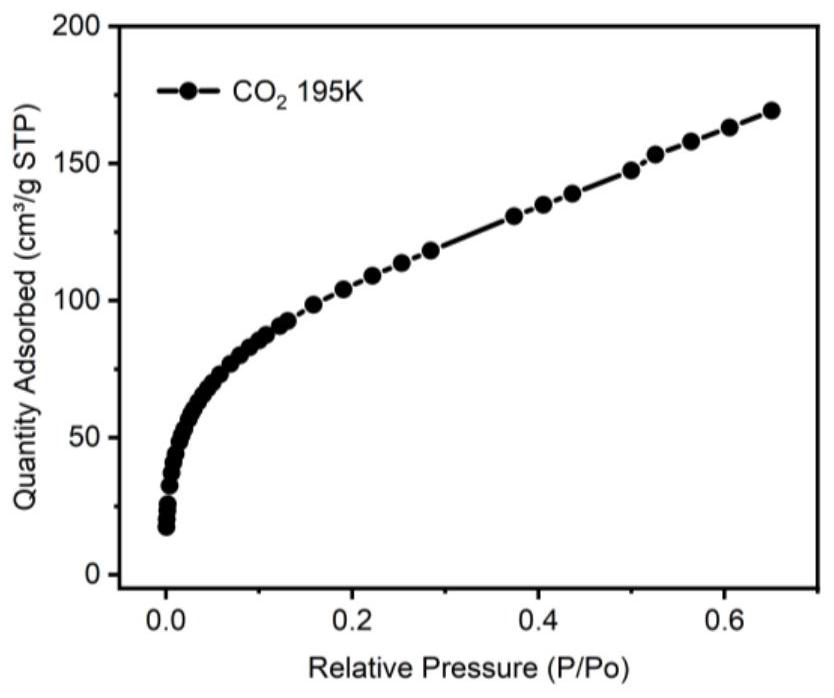
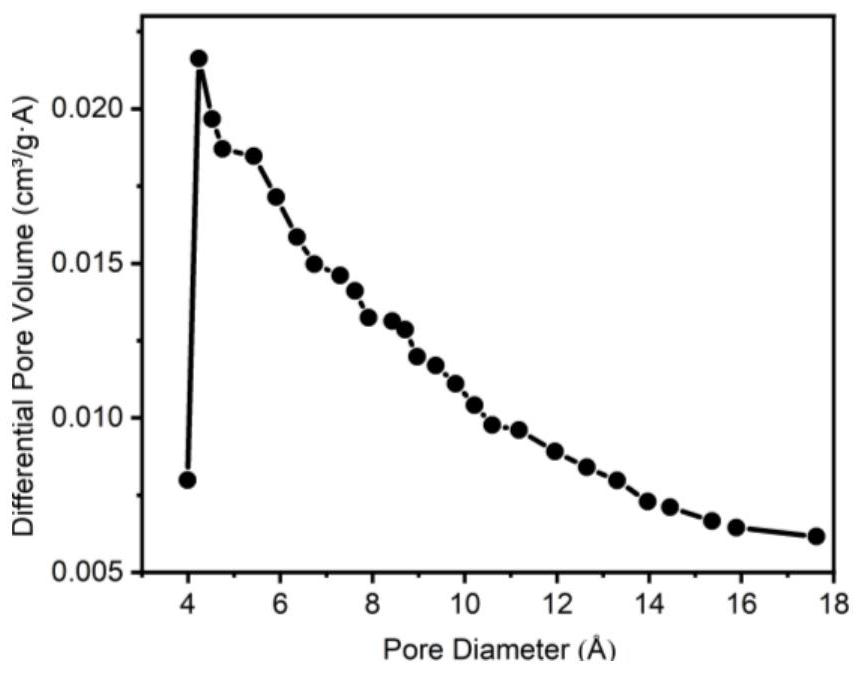
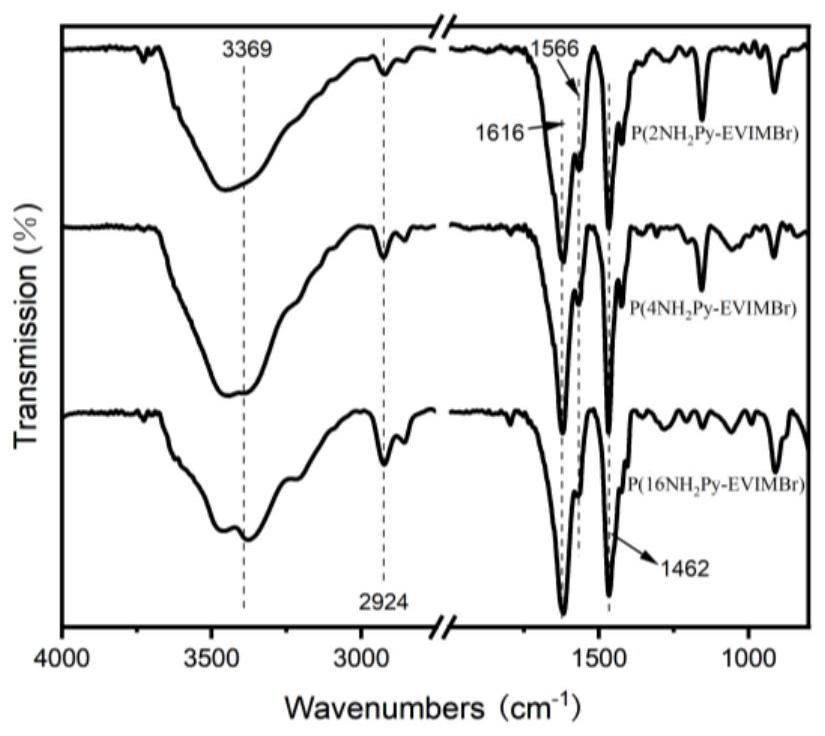
Image
Examples
Embodiment 1
[0054] Weigh 1.0 g of 2-amino-3,5-dibromopyridine, 1.32 g of potassium vinyl trifluoroborate, 1.6 g of potassium carbonate, and 0.116 g of triphenylphosphonium palladium, and add them to a mixture of 25 ml of toluene, 25 ml of tetrahydrofuran and 25 ml of water. The mixture was mixed and dissolved, and then heated to 90° C. under a nitrogen atmosphere to react for 48 hours. The obtained product was extracted with ethyl acetate, washed with brine, and then dried over anhydrous sodium sulfate. Crude product was obtained after distillation under reduced pressure. The crude product was then purified by flash chromatography using n-hexane / ethyl acetate (1:1) and 3% v / v triethylamine as eluent to give a pale yellow powdery solid, the basic functionalized crosslinker The molecular structure of 2-amino-3,5-divinylpyridine is shown in formula (I), and the yield is about 70%.
[0055]
[0056] Weigh 1.0 g of 4-amino-3,5-dibromopyridine, 1.32 g of potassium vinyl trifluoroborate, 1....
Embodiment 2
[0061] In a 50mL Schlenk storage bottle, weigh 1.00g of the basic functionalized crosslinking agent 2-amino-3,5-divinylpyridine obtained in Example 1, 0.087g of 1-vinyl-3-ethylimidazolium bromide (mol ratio 16:1) and 0.022g azobisisobutyronitrile, dissolved in 14mL mixed solution of ethyl acetate, ethanol and water (volume ratio 8:4:2), mixed and dissolved under stirring, and then heated to 80°C React for 24 hours. After cooling at room temperature, the obtained product was filtered, washed with water and ethyl acetate, and then vacuum-dried at 100° C. for 24 hours to obtain a yellow solid powder with a yield of about 75%.
Embodiment 3
[0063] In a 50mL Schlenk storage bottle, weigh 1.00g of the basic functionalized crosslinking agent 4-amino-3,5-divinylpyridine obtained in Example 1, 0.087g of 1-vinyl-3-ethylimidazolium bromide (mol ratio 16:1) and 0.022g azobisisobutyronitrile, dissolved in 14mL mixed solution of ethyl acetate, ethanol and water (volume ratio 8:4:2), mixed and dissolved under stirring, and then heated to 80°C The reaction was carried out for 8 hours. After cooling at room temperature, the obtained product was filtered, washed with water and ethyl acetate, and then vacuum-dried at 80° C. for 24 hours to obtain a yellow solid powder with a yield of about 76%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com