Pyrrole-2-aldehyde compound as well as preparation method and application thereof
An aldehyde compound and pyrrole technology, applied in the field of pharmacy, can solve the problems such as no reports on the activity of monomer components, and achieve the effects of low toxicity, increased benefit, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
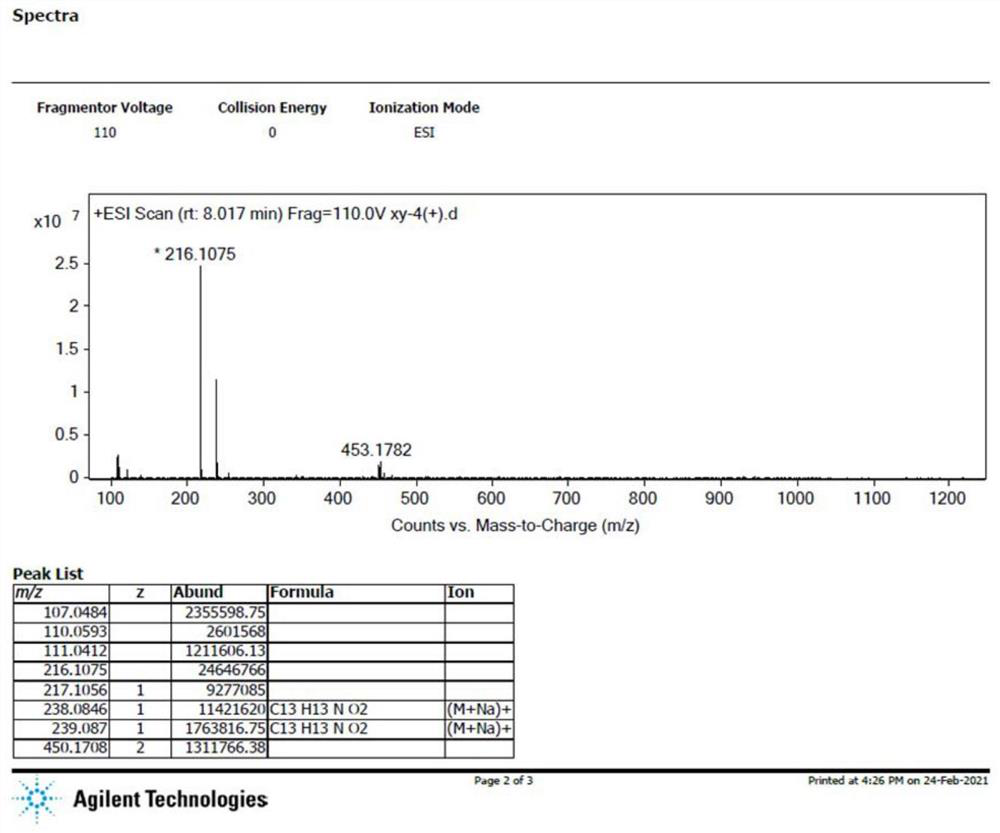
[0102] Extraction, Separation and Structure Identification of Pyrrole-2-aldehyde Compounds
[0103] Take 10 kg of Moringa oleifera seeds and grind them, add 8 times the weight of purified water for ultrasonic extraction three times, each time for 1 hour, combine the extracts, concentrate, filter, and adsorb the filtrate on a PHD-300 macroporous resin column, wash with water, 50% ethanol and 90% ethanol in sequence The water, 50% ethanol and 90% ethanol eluents were collected respectively, and concentrated to obtain fluid extracts of three eluted parts. Among them, about 1.0kg of the water-washed liquid extract was applied to MCI resin, and then eluted with water, 50% ethanol and 90% ethanol in sequence, and the eluents of water, 50% ethanol and 90% ethanol were collected respectively, and the eluent was collected, concentrated, and decompressed. Dry to obtain the extract of the three eluted parts of the MCI column. Wherein MCI50% ethanol eluted extract 104g, put on the revers...
Embodiment 2
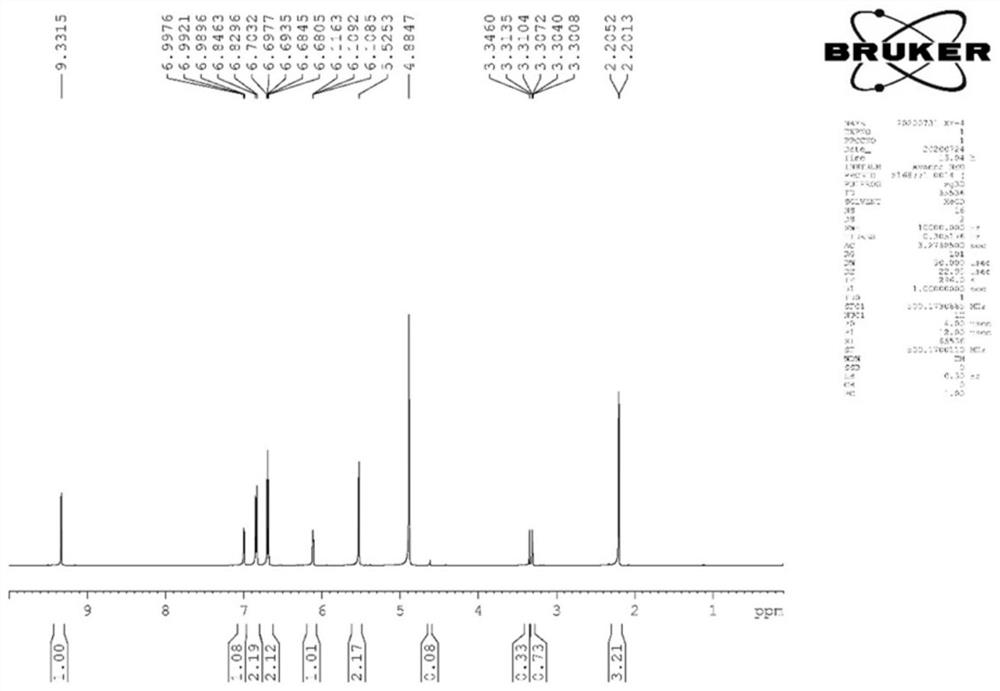
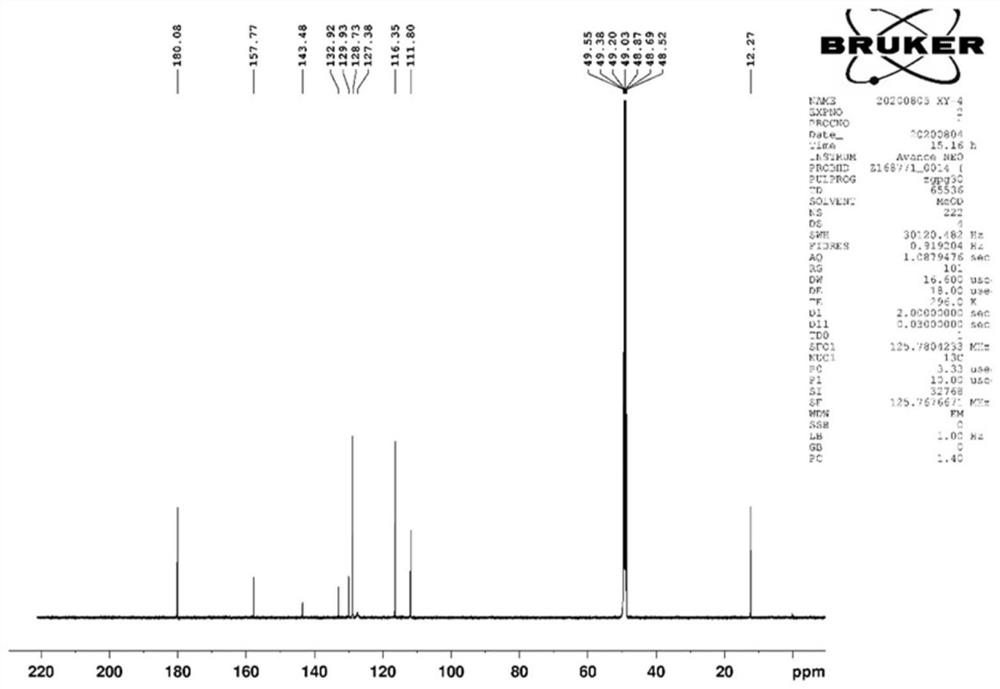
[0142] Synthesis of Pyrrole-2-aldehyde Compounds
[0143] First take KOH, pyrrole-2-aldehyde components, and benzyl chloride with a molar ratio of 1:1:1, add 5 mL of DMSO, heat and stir in an oil bath at 65°C for 24 hours, mix the sample with silica gel after the reaction is completed, and separate it with a silica gel column. : 1 petroleum ether: ethyl acetate eluted, collected the fractions, plated and combined, purified to obtain the reaction product, the yield was 60%-80%, and synthesized to obtain the product XYHC-1, XYHC-2. The general reaction formula is as follows:
[0144]
[0145] Among them, R 2 is hydrogen, methyl, hydroxymethyl, aliphatic ether group, nitrogen-containing heterocycle, sulfur-containing heterocycle, halogenated benzene ring or halogenated benzyl; R 3 is hydrogen, methyl, ethyl, benzene ring, benzyl, halogenated benzene ring, halogenated benzyl, N-containing heterocycle or sulfur-containing heterocycle. After identification, the structure of th...
Embodiment 3
[0153] Effects of pyrrole-2-aldehyde compounds on the scavenging rate of oxidative stress free radicals
[0154] 1. DPPH (1,1-Diphenyl-2-picrylhydrazyl radical) is 1,1-diphenyl-2-picrylhydrazyl radical. In the molecule, due to the presence of multiple electron-withdrawing -NO 2 And the large π bond of the benzene ring, so the nitrogen radical can exist stably. When DPPH free radicals are removed, the absorbance A value at the maximum absorption wavelength of 519nm decreases accordingly.
[0155] Preparation and handling of DPPH solvent
[0156] Preparation of DPPH test solution: Dissolve 1 mg of DPPH in about 20 mL of solvent (ethanol, 95% ethanol or methanol), ultrasonicate for 5 minutes, and shake fully to make the upper and lower parts uniform. Take 1mL DPPH solution, measure A value at 519nm, and make A=1.2-1.3 the best. The DPPH solution is best kept away from light and used up within 3.5 hours.
[0157] Determination of experimental samples
[0158] The pyrrole-2-a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com